Celiac disease, short stature and growth hormone deficiency
Introduction
Celiac disease (CD) is more than a gastrointestinal disease. After the original description in 1888 by Gee, and its association with gluten from the diet in the 1940s by Dicke [review in (1)], the clinical presentation of the most common genetically-based disease associated with food intolerance goes beyond intestinal manifestations. Up to 4% of children seeking medical care due to short stature might have CD, many without gastrointestinal symptoms (2). Improvement in growth velocity and normalization of height is observed with a strict adherence to a gluten-free diet (GFD). However, few children do not show catch-up growth after an extended period of diet. For these children, the possibility of growth hormone (GH) deficiency should be considered. In this review, we discuss the interactions between GH secretion and the gastrointestinal tract in children with CD.
Celiac disease
CD is defined as an immune-mediated enteropathy, with characteristic changes in the intestinal histology. It is characterized as a permanent sensitivity to gluten, and it occurs in genetically susceptible individuals (HLA class II haplotype DQ2 or DQ8). The classic form of CD in children consists of gastrointestinal symptoms starting between 6 and 24 months of age, after the introduction of gluten in the diet. Patients present with malabsorption syndrome that includes chronic diarrhea, poor weight gain or weight loss, vomiting, abdominal distension, abdominal pain, anorexia (3).
The clinical manifestation of patients with CD has changed dramatically over the recent decades, with diarrheal or classic presentations becoming less common. The main presentation seen nowadays in children include recurrent abdominal pain and growth issues (4). Gastrointestinal symptoms in older children include recurrent diarrhea or more nonspecific symptoms like constipation, nausea and vomiting, abdominal pain, bloating, weight loss or even obesity (5,6). Many patients present initially with nongastrointestinal manifestations of CD (Table 1). These manifestations include osteopenia and osteoporosis, which increase the risk of bone fractures (triggered even by mild traumatic injuries). In these patients, fracture risk can be reversed by GFD (7,8). Unexplained iron deficiency anemia resistant to oral iron supplementation is the most common nongastrointestinal manifestation in adults, and has been reported in children. Dermatitis herpetiformis is a skin manifestation of CD. Dental enamel defects of permanent teeth, epilepsy with occipital calcification and arthritis were also described. Elevated levels of transaminases of unclear etiology can be seen (5).
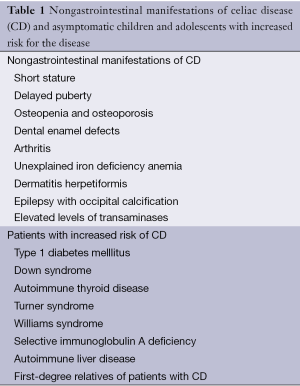
Full table
Delayed puberty or short stature can be the initial presenting manifestation. The risk of CD in patients with isolated stunted growth or short stature has been calculated as 10-40% (9,10). Impaired growth in children with CD results mainly from nutritional deficits, and withdrawal of gluten from the diet is frequently associated with a marked improvement of linear growth within two years (11). In our unit hospital, from a group of 40 children with the diagnosis of CD, 20% presented with classical CD, 60% had oligosymptomatic CD, 17.5% had atypical CD (15% with short stature), and 2.5% had asymptomatic CD. Some centers still describe a high prevalence of classical CD. This probably relates to referral bias. Our hospital is a referral center for growth disorders, one of the main complaints in children with CD. Most of these children undergo screening evaluation through serological markers. Asymptomatic children and adolescents with increased risk of CD are first-degree relatives with CD, children with type 1 diabetes mellitus, Down syndrome, autoimmune thyroid disease, Turner syndrome, Williams syndrome, selective immunoglobulin A deficiency, and autoimmune liver disease (12).
CD is characterized by the presence of autoantibodies generated in response to gluten exposition in genetically susceptible individuals. Present guidelines are in agreement regarding which test is the best for initial serological screening. Measurement of antibodies against tissue transglutaminase (tTG) is the most reliable and cost-effective test for CD. Measurement of antibodies against endomysium (EMA) is as accurate as tTG, but it is an immunofluorescence test, and is therefore observer-dependent and more subject to interpretation error and added cost (3). A third antibody, produced against the deamidated gliadin peptides may be used as additional test in patients who are negative for other CD-specific antibodies, but in whom clinical symptoms raise a strong suspicion of CD, especially if they are younger than 2 years of age (12).
Serological screening is initially recommended for patients with suspected CD. Those with positive tests should undergo small intestinal biopsy to confirm the diagnosis according to the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) (3). Recent guidelines published by the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) suggest that biopsies may not be necessary for patients with typical symptoms of CD and antibodies tTG above ten times the upper limit of normal, together with positive antibodies to EMA and an allele for the HLA-DQ2 or DQ8 haplotype. For asymptomatic children at increased risk of CD, the diagnosis is based on positive serology and positive histology findings on biopsies. Both serology and biopsy should be performed while on a gluten-containing diet (12).
The only treatment currently available for CD is strict adherence to a GFD for life, which results in a complete return to health (3,12).
Failure to thrive in children with CD
Failure to thrive and short stature are common findings in children with CD and gastrointestinal symptoms (13,14). Early in the 1970s, the finding of short stature as the only manifestation of CD became more frequently recognized (15,16). Philip et al. found short estature in 25%, delayed puberty in 11% and both in 20% of 36 patients as the initial complaint. After complete evaluation, 58% had short stature and 31% had delayed puberty (17). Adherence to a GFD generally leads to catch-up growth (14,18,19). Usually, weight fully catches-up 6 months after the start of a GFD, and height catches-up 2 years later (20,21). However, some children with growth failure do not improve growth after starting a GFD, despite reversion to seronegativity for antibodies. It has been reported that a GFD will be successful if at diagnosis there is a delay of bone age, and in the first year of diet there is an evident catch-up growth (22). When catch-up growth does not occur, it may be due to an associated GH deficiency (23).
In the group studied by Bosio et al. (24), patients showed an increased height velocity during the first 3 years while on a GFD, with maximum growth velocity occurring during the first year, but the catch-up growth was incomplete over 3 years. The 12 patients who completed pubertal development reached their target height, independent of the duration of the GFD. The final height seemed influenced mainly by familial characteristics; height was below the third percentile in 31% of parents examined.
Recently, Bozzola et al. (25) described the case of a girl presenting with stunted growth and malnourishment, without any signs of gastrointestinal, renal or endocrine disorders. She was evaluated for CD, but resulted negative for anti-tTG antibodies. At the age of 4.1 years, she exhibited iron deficiency anaemia despite repeated iron supplementation, with persistent reduced height, body mass index (BMI), growth velocity and delayed bone age. The CD screening was repeated; very high anti-tTG-IgA and -IgG values were found, and a duodenal biopsy was positive. After only four months of GFD, her growth velocity increased from 4.83 cm/year (–1.79 SDS) to 6.53 cm/year (–0.15 SDS).
The pathogenesis of CD-associated short stature is still unclear. The damages to the small intestinal mucosa, with consequent nutritional deficits, are responsible for impaired growth. These children usually present with reduction of insulin-like growth factor 1 (IGF1), IGF2 and insulin-like growth factor binding protein 3 (IGFBP-3), increase of IGFBP-2 and IGFBP-1 levels, and a blunted GH response to pharmacological stimuli. A significant inverse association was found between the duration of gluten exposure and IGF1 levels, and a significant reduction in IGF1 levels was observed after prolonged gluten exposure, before growth failure (21). These findings correspond with those observed in chronically malnourished children (21,26). Reevaluation of IGF1 levels while on a GFD showed rapid reversal, with an increase in GH binding protein (GHBP), IGF1, IGF2 and IGFBP-3 levels, and a decrease in IGFBP-1. These changes suggest an improvement in the sensitivity to GH, reflecting the recovery towards a normally functioning somatotrophic axis (21,27).
A summary with possible causes of short stature in CD patients are presented in Table 2.

Full table
GH deficiency in children with CD
Rarely, it has been shown that poor catch-up growth response to GFD is due to a coexistence of GH deficiency (11,22,23,28). Out of 7066 children with short stature, Giovenale et al. (2) found 16 (0.23%) subjects with both GH deficiency and CD, and two of them with probable congenital GH deficiency (23). CD is an autoimmune disease often associated with other endocrine and non-endocrine autoimmune diseases (12). In fact, CD is actually seen as an immune-mediated systemic disorder. Iughetti et al. (28) have demonstrated the presence of high titers of antipituitary antibodies in CD children without catch-up growth after GFD, suggesting an autoimmune form of hypophysitis in these patients. The Italian Autoimmune Hypophysitis Network Study also reported high titers of antipituitary antibodies associated with height impairment in newly diagnosis celiac patients (29). However, Ferrante et al. (30) could not confirm the presence of antipituitary antibodies in adult patients with CD and pituitary dysfunction. More recently, Aguado et al. (31) showed that antipituitary autoantibodies can also be detected in patients with gastroenteropathies other than CD, but without a direct relationship with growth development nor with IGF1 levels, suggesting that another feature could be responsible for different clinical manifestations between CD and nongluten-related enteropathies.
The pathophysiological mechanisms leading to the absence of catch-up growth after GFD in some children with CD are not totally understood yet. Presumably, these mechanisms can be explained by blunted GH and IGF1 secretion, and these children might benefit from GH treatment (32). GH-treated patients with CD and GH deficiency can reach final adult height close to their genetic potential (33).
Interactions between the endocrine system and the gastrointestinal tract
CD is an immune-mediated disease that occurs in individuals intolerant to gluten. It is generally accepted that it is a T-cell-mediated disease, in which gliadin-derived peptides are deaminated by tTG and presented by antigen presenting cells to lamina propria T helper (Th) lymphocytes. Pro-inflammatory cytokines are released with activation of intraepithelial lymphocytes and consequent histologic alterations. Cytokines are elevated and correlated with disease activity, characterizing the inflammatory aspect of this disease (34). In view of the inflammatory and nutritional aspects of CD, some aspects of the physiological system that regulates body weight, fat stores, energy intake and energy expenditure should be taken in consideration. This regulatory system is formed by multiple interactions between the gastrointestinal tract, adipose tissue, the endocrine and the central nervous systems.
Leptin in CD
Leptin is a peptide hormone produced by the ob gene and secreted mainly by adipocytes. A number of other cell types also produce leptin, including gastric and colonic epithelial cells, and T-cells, especially during acute inflammation. The production of leptin is higher in subcutaneous fat than in visceral fat, and in the blood, leptin levels correlate directly with the amount of body fat. The secretion of leptin is reduced during periods of fasting and increased after meals. Leptin stimulates anorexigenic neurons and inhibits orexigenic neurons that express neuropeptide Y and Agouti-related peptide. Leptin is the main catabolic adiposity signal, which actions result in reduced food intake, increased energy expenditure and weight loss (35,36). Leptin receptor belongs to the type I cytokine receptor family and intestinal mucosa contains leptin receptors. It was postulated that direct leptin signaling in the intestine might be involved in the regulation of nutrient absorption and intestinal motility. Furthermore, leptin is involved in immune regulation (37).
Children with CD usually present with weight loss and malnutrition, which can be severe or mild. Maggio et al. (38) reported low leptin levels in 14 children with CD, 71% of them with values below –2 SD score for gender and age. A direct correlation with weight and BMI was found, but the physiological association of leptin with age described in healthy individuals was lost. Leptin levels were lower in patients with severe mucosal atrophy and rose after 6-12 months of GFD. The association with the histopathologic findings was not confirmed by Ertekin et al. (39), despite similar findings of low leptin levels in CD children. In contrast, in children with CD and satisfactory nutritional status at the diagnosis, comparable with the general population, leptin levels were also comparable with that from controls, reflecting the similarity of fat mass in both groups (40).
Few data is available on leptin and inflammatory activity in children with CD. When analyzing both leptin and the pro-inflammatory cytokine tumour necrosis factor (TNF) in children with CD, the low leptin levels were confirmed in active CD, but without correlation with BMI. This correlation was present only for those patients in remission. TNF receptor (TNFr-1) levels were higher in patients with active CD. The authors suggested that leptin does not contribute to anorexia and failure to thrive in patients with CD; in contrast, the TNF system could be involved (41). It is well know that leptin induces growth by regulating the energy levels of the organism and by stimulating the production and secretion of GH (42). However, the effect of low leptin levels on GH secretion in children with CD is not known.
Ghrelin
Ghrelin is a peptide hormone that has been isolated from stomach. It is found mostly in endocrine cells in the oxyntic mucosa, but small amounts are also found in the small intestine and arcuated nucleus of the hypothalamus (43,44). Ghrelin receptors are expressed in all parts of the gastrointestinal tract (45). Ghrelin has several functions. Besides its GH-releasing effect in the pituitary, it stimulates appetite, reduces fat utilization, affects body composition, induces hyperglycemia and can override the anoretic action of leptin (43,46).
In healthy children, ghrelin levels decrease with age and puberty, correlate negatively to IGF1 and IGFBP-3 and positively with IGFBP-1, effects that lower tissue availability of IGF1 (47). The authors suggested that the reduction of ghrelin with age and during puberty with higher IGF1 levels contribute to growth spurt during puberty. During starvation, ghrelin levels increase as a response of the neuronal circuits to induce a positive energy balance. Ghrelin reduces energy expenditure through action on the hypothalamic-pituitary-thyroid axis, decreasing TSH levels and stimulating the hypothalamic-pituitary-adrenal axis. GH secretion also increases (48). Since ghrelin is an endogenous agonist at the GH secretagogue receptor, this could be one explanation for the high GH levels in anorexic patients and for the decreased GH secretion in the obese ones (49).
Ghrelin also has a role in immune and inflammatory responses and gastrointestinal motility. Since gastrointestinal diseases exhibit gastrointestinal dysmotility and/or inflammation, ghrelin might have clinical implications in these diseases (50). Serum ghrelin levels are higher in adults with CD at the diagnosis compared with controls, decreasing with GFD; an inverse association between ghrelin and BMI is observed only after appropriate diet (51,52). In children with CD, biopsies taken from distal duodenum showed higher number of ghrelin-positive cells compared with controls. The density of ghrelin-positive cells did not correlate with age, BMI or clinical presentation (53). In a study involving 36 children with CD, serum ghrelin levels were higher in children with CD compared with controls, and negatively correlated with BMI (50). No significant difference was found between children with classic CD (chronic diarrhea, abdominal distention and malnutrition) and children with short stature only. After 6 months of GFD, ghrelin levels decreased, but remained higher than those controls (46). Mean serum ghrelin levels were not different in prepubertal and pubertal children (46,54), boys and girls (46,47).
Taken together, these results show an overproduction of ghrelin in children with CD and suggest that the mucosal inflammation is not a major factor affecting the level of circulating ghrelin. The impaired nutritional status increases ghrelin levels, which return to normal after GFD and weight recovery. The growth failure in CD children despite high ghrelin levels probably occurs because the increased ghrelin level does not act as a direct growth-promoting hormone, but suggest a complex network regulating appetite and energy metabolism regulation.
Conclusions
CD is more than a gastrointestinal disease. CD is a common immunological disorder that can present at any age with classical or atypical symptoms. Nutritional status can vary from undernourished to obesity or only micronutrient deficiencies. Inadequate intake of some micronutrients can continue after a strict adherence to a GFD. Although we have come a long way to understand the mechanisms of nutrition, energy regulation and hormone secretion in children with CD, more studies are still necessary to come to a full understanding.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Guandalini S, Assiri A. Celiac disease: a review. JAMA Pediatr 2014;168:272-8. [PubMed]
- Giovenale D, Meazza C, Cardinale GM, et al. The prevalence of growth hormone deficiency and celiac disease in short children. Clin Med Res 2006;4:180-3. [PubMed]
- Hill ID, Dirks MH, Liptak GS, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2005;40:1-19. [PubMed]
- Reilly NR, Fasano A, Green PH. Presentation of celiac disease. Gastrointest Endosc Clin N Am 2012;22:613-21. [PubMed]
- Fasano A. Clinical presentation of celiac disease in the pediatric population. Gastroenterology 2005;128:S68-73. [PubMed]
- McGowan KE, Castiglione DA, Butzner JD. The changing face of childhood celiac disease in north america: impact of serological testing. Pediatrics 2009;124:1572-8. [PubMed]
- Vasquez H, Mazure R, Gonzalez D, et al. Risk of fractures in celiac disease patients: a cross-sectional, case-control study. Am J Gastroenterol 2000;95:183-9. [PubMed]
- Mora S, Barera G, Beccio S, et al. A prospective, longitudinal study of the long-term effect of treatment on bone density in children with celiac disease. J Pediatr 2001;139:516-21. [PubMed]
- Tümer L, Hasanoglu A, Aybay C. Endomysium antibodies in the diagnosis of celiac disease in short-statured children with no gastrointestinal symptoms. Pediatr Int 2001;43:71-3. [PubMed]
- van Rijn JC, Grote FK, Oostdijk W, et al. Short stature and the probability of coeliac disease, in the absence of gastrointestinal symptoms. Arch Dis Child 2004;89:882-3. [PubMed]
- Nemet D, Raz A, Zifman E, et al. Short stature, celiac disease and growth hormone deficiency. J Pediatr Endocrinol Metab 2009;22:979-83. [PubMed]
- Husby S, Koletzko S, Korponay-Szabó IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136-60. [PubMed]
- Young WF, Pringle EM. 110 children with coeliac disease, 1950-1969. Arch Dis Child 1971;46:421-36. [PubMed]
- Meazza C, Pagani S, Laarej K, et al. Short stature in children with coeliac disease. Pediatr Endocrinol Rev 2009;6:457-63. [PubMed]
- Verkasalo M, Kuitunen P, Leisti S, et al. Growth failure from symptomless celiac disease. A study of 14 patients. Helv Paediatr Acta 1978;33:489-95. [PubMed]
- Groll A, Candy DC, Preece MA, et al. Short stature as the primary manifestation of coeliac disease. Lancet 1980;2:1097-9. [PubMed]
- Philip R, Patidar P, Saran S, et al. Endocrine manifestations of celiac disease. Indian J Endocrinol Metab 2012;16:S506-8. [PubMed]
- Barr DG, Shmerling DH, Prader A. Catch-up growth in malnutrition, studied in celiac disease after institution of gluten-free diet. Pediatr Res 1972;6:521-7. [PubMed]
- Damen GM, Boersma B, Wit JM, et al. Catch-up growth in 60 children with celiac disease. J Pediatr Gastroenterol Nutr 1994;19:394-400. [PubMed]
- Boersma B, Wynne HJ, Wit JM. A mathematical model describing catch-up growth in celiac disease. Acta Paediatr 1994;83:1097-9. [PubMed]
- Boersma B, Houwen RH, Blum WF, et al. Catch-up growth and endocrine changes in childhood celiac disease. Endocrine changes during catch-up growth. Horm Res 2002;58 Suppl 1:57-65. [PubMed]
- Salardi S, Cacciari E, Volta U, et al. Growth and adult height in atypical coeliac patients, with or without growth hormone deficiency. J Pediatr Endocrinol Metab 2005;18:769-75. [PubMed]
- Bozzola M, Giovenale D, Bozzola E, et al. Growth hormone deficiency and coeliac disease: an unusual association? Clin Endocrinol (Oxf) 2005;62:372-5. [PubMed]
- Bosio L, Barera G, Mistura L, et al. Growth acceleration and final height after treatment for delayed diagnosis of celiac disease. J Pediatr Gastroenterol Nutr 1990;11:324-9. [PubMed]
- Bozzola M, Bozzola E, Pagani S, et al. Late diagnosis of celiac disease in an asymptomatic infant with growth failure. Ital J Pediatr 2014;40:4. [PubMed]
- Ketelslegers JM, Maiter D, Maes M, et al. Nutritional regulation of the growth hormone and insulin-like growth factor-binding proteins. Horm Res 1996;45:252-7. [PubMed]
- Eichler I, Frisch H, Granditsch G. Growth failure and insulin-like growth factor (IGF-I) in childhood celiac disease. Klin Wochenschr 1991;69:825-9. [PubMed]
- Iughetti L, De Bellis A, Predieri B, et al. Growth hormone impaired secretion and antipituitary antibodies in patients with coeliac disease and poor catch-up growth after a long gluten-free diet period: a causal association? Eur J Pediatr 2006;165:897-903. [PubMed]
- Delvecchio M, De Bellis A, Francavilla R, et al. Anti-pituitary antibodies in children with newly diagnosed celiac disease: a novel finding contributing to linear-growth impairment. Am J Gastroenterol 2010;105:691-6. [PubMed]
- Ferrante E, Giavoli C, Elli L, et al. Evaluation of GH-IGF-I axis in adult patients with coeliac disease. Horm Metab Res 2010;42:45-9. [PubMed]
- Aguado R, Fernández S, Estévez OA, et al. Antiadenohypophysis autoantibodies in patients with nongluten-related gastroenteropathies. J Clin Lab Anal 2014;28:59-62. [PubMed]
- Giovenale D, Meazza C, Cardinale GM, et al. Growth hormone treatment in prepubertal children with celiac disease and growth hormone deficiency. J Pediatr Gastroenterol Nutr 2007;45:433-7. [PubMed]
- Meazza C, Pagani S, Messini B, et al. Coeliac children treated for growth hormone deficiency reach normal final height. Clin Endocrinol (Oxf) 2011;74:791-2. [PubMed]
- Manavalan JS, Hernandez L, Shah JG, et al. Serum cytokine elevations in celiac disease: association with disease presentation. Hum Immunol 2010;71:50-7. [PubMed]
- Mackey-Lawrence NM, Petri WA Jr. Leptin and mucosal immunity. Mucosal Immunol 2012;5:472-9. [PubMed]
- Boguszewski CL, Paz-Filho G, Velloso LA. Neuroendocrine body weight regulation: integration between fat tissue, gastrointestinal tract, and the brain. Endokrynol Pol 2010;61:194-206. [PubMed]
- Paz-Filho G, Mastronardi C, Franco CB, et al. Leptin: molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq Bras Endocrinol Metabol 2012;56:597-607. [PubMed]
- Maggio MC, Corsello G, Iacono G, et al. Gluten-free diet impact on leptin levels in asymptomatic coeliac adolescents: one year of follow-up. Horm Res 2007;67:100-4. [PubMed]
- Ertekin V, Orbak Z, Selimoglu MA, et al. Serum leptin levels in childhood celiac disease. J Clin Gastroenterol 2006;40:906-9. [PubMed]
- Aurangzeb B, Leach ST, Lemberg DA, et al. Nutritional status of children with coeliac disease. Acta Paediatr 2010;99:1020-5. [PubMed]
- Blanco Quirós A, Arranz Sanz E, Garrote Adrados JA, et al. The tumor necrosis factor system and leptin in coeliac disease. An Esp Pediatr 2001;55:198-204. [PubMed]
- Gat-Yablonski G, Phillip M. Leptin and regulation of linear growth. Curr Opin Clin Nutr Metab Care 2008;11:303-8. [PubMed]
- Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656-60. [PubMed]
- Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000;141:4255-61. [PubMed]
- Takeshita E, Matsuura B, Dong M, et al. Molecular characterization and distribution of motilin family receptors in the human gastrointestinal tract. J Gastroenterol 2006;41:223-30. [PubMed]
- Selimoglu MA, Altinkaynak S, Ertekin V, et al. Serum ghrelin levels in children with celiac disease. J Clin Gastroenterol 2006;40:191-4. [PubMed]
- Whatmore AJ, Hall CM, Jones J, et al. Ghrelin concentrations in healthy children and adolescents. Clin Endocrinol (Oxf) 2003;59:649-54. [PubMed]
- Horvath TL, Diano S, Sotonyi P, et al. Minireview: ghrelin and the regulation of energy balance--a hypothalamic perspective. Endocrinology 2001;142:4163-9. [PubMed]
- Inui A, Asakawa A, Bowers CY, et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J 2004;18:439-56. [PubMed]
- El-Salhy M. Ghrelin in gastrointestinal diseases and disorders: a possible role in the pathophysiology and clinical implications Int J Mol Med 2009;24:727-32. [PubMed]
- Peracchi M, Conte D, Terrani C, et al. Circulating ghrelin levels in celiac patients. Am J Gastroenterol 2003;98:2474-8. [PubMed]
- Lanzini A, Magni P, Petroni ML, et al. Circulating ghrelin level is increased in coeliac disease as in functional dyspepsia and reverts to normal during gluten-free diet. Aliment Pharmacol Ther 2006;23:907-13. [PubMed]
- Jarocka-Cyrta E, Kasacka I, Kaczmarski M. The ghrelin-positive cells number is increased in duodenum in children with celiac disease. J Endocrinol Invest 2010;33:165-70. [PubMed]
- Bellone S, Castellino N, Broglio F, et al. Ghrelin secretion in childhood is refractory to the inhibitory effect of feeding. J Clin Endocrinol Metab 2004;89:1662-5. [PubMed]


