Gastroenteropancreatic neuroendocrine tumors (GEP-NETs)
Introduction
The gut is similar to an endocrine organ, and produces several hormones and substances, including substances from neuronal cells. These hormones and substances may have endocrine, paracrine, autocrine and neurocrine roles (1). The endocrine system of the gut contributes to the regulation of mechanical, chemical and enzymatic processes of digestion, control of post-absorptive processes involved in the assimilation of digested food, regulation of food intake, central nervous system (CNS) feedback, and growth and development of the gut (2-5). Most gastrointestinal peptides are released in response to meals, and meal type and size are important in the degree of release. Gastrointestinal peptides act by binding to transmembrane G protein-coupled receptors, and circulating enzymes usually break down gut peptides, which are metabolized and excreted by the liver and kidney (1).
Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are tumors derived from neuroendocrine cells and they can occur anywhere along the gut (6). GEP-NETs are classified with regard to the type of clinical syndrome present. Generally they share several common features including various aspects of pathology, natural history, treatment options, localization procedures, and treatment of metastatic tumors. Normal physiological regulations of hormones are lost in the GEP-NETs and the tumor release hormones autonomously (7). The majority of GEP-NETs are sporadic however they can be also part of familiar syndromes such as multiple endocrine neoplasia type 1 (MEN1) syndrome, von-Hippel-Lindau disease, tubero sclerosis and neurofibromatosis type 1 (8).
Here we will discuss the epidemiology, pathogenesis, clinical features, diagnosis and treatment of rare GEP-NETs including insulinoma, glucagonoma, gastrinoma, vipoma, somatostatinoma and carcinoids tumor.
Epidemiology
GEP-NETs are uncommon, with an incidence of 2.5-5 cases per 100,000 (9). Siegfried Oberndorfer coined the name ‘Karzinoide’ to describe submucosal tumors in the small bowel which followed an indolent course when compared to adenocarcinoma in 1907 and since then major changes have been made in the classification of GEP-NETs (10). William J. Mayo performed the first (unsuccessful) resection of a malignant, metastatic pancreatic insulinoma in 1926. Robert Zollinger and Edwin Ellison were the first to describe a functionally active pancreatic gastrinoma in 1955. The first description of a glucagon-secreting GEP-NETs was made by Malcolm H. McGavran, Roger H. Unger and a group of surgeons and pathologists in 1966 (11).
Due to increased availability of advanced endoscopic and radiological imaging, the diagnosis of benign and incidentally identified lesions has also increased over the past decades. In terms of symptoms and outcome, clinical behavior of GEP-NETs varies strikingly. For example, overall 5-year survival for GEP-NETs varies from 30% for those that are non-functioning and clinically silent tumors, to 97% for benign insulinomas. Therefore the idea of GEP-NETs are slow growing should be reconsidered (10,12). Inconsistency of nomenclature and classification of GEP-NETs is the major limitation in clarifying the exact epidemiology of GEP-NET (13). Surveillance, epidemiology, and end results (SEER) database shows that over 30 years the age-adjusted incidence of carcinoids of the small intestine and digestive system has increased by 460% and 720%, respectively. However, overall 5-year survival for carcinoid tumours of the small intestine is about 60% in USA and this proportion has not changed substantially since 1973 (10).
Insulinomas were reported to be the most common GEP-NETs in some older series with an incidence in various series of 0.8-0.9 per million population per year, whereas gastrinomas were reported to occur in 0.1-0.4 per million per year. However, in subsequent studies, gastrinomas were as common as insulinomas (1-3 new cases/year/million population). Incidence of the remaining symptomatic GEP-NETs is less than 0.5 per million per year for each (14-16).
Pathogenesis and tumor biology
Neuroendocrine tumorigenesis follows a progression pathway of hyperplasia, dysplasia, neoplasia (well-differentiated, progressing to lesser degrees of differentiation), and metastasis similar to that of other tumors. But unlike other tumors, their progression through these stages is slow. When tumors become less differentiated, they develop the potential to secrete other peptides. This may cause a different clinical tumor syndrome (1).
They include a heterogeneous family of neoplasms with a broad and complex spectrum of clinical behavior. GEP-NETs have conservatively been sectioned into foregut (esophagus, stomach, proximal duodenum, liver and pancreas), midgut (distal duodenum ileum, jejunum, ascending colon and proximal two thirds of transverse colon) and hindgut tumors (distal third of transverse colon, descending colon, sigmoid colon and rectum) (6). Except for nonfunctioning GEP-NETs the extensive release of hormones by the tumor into the circulation is the principal clinical manifestations of the endocrine tumor in each case. These tumors can be subdivided into functional or nonfunctional depending on if a clinical syndrome caused by an ectopically released hormone is present or not. Hormones and peptides [chromogranin, neurotensin, pancreatic polypeptide (PP), or neuron-specific enolase] are frequently ectopically released by nonfunctional GEP-NETs but these peptides do not cause distinct clinical syndromes (7). Because nonfunctioning GEP-NETs are indolent and they may escape clinical detection, at the time of diagnosis they often are larger and more frequently are malignant (8).
Neuroendocrine tumors (NETs) produce hormones which are not normally synthesized in the adult pancreas, and they frequently contain ductular structures. Therefore, the idea of originating from an immature stem cell is thought by some (7). In addition, since endocrine cells bud off from ductules during ontogenesis of the pancreas, and ductular structures are present in these tumors, it has been hypothesized that these cells may originate from ducts. A study in patients with MEN1 syndrome, 80-100% of whom developed GEP-NETs, obtained evidence that the GEP-NETs developed from ducts. Whereas another study claimed they originate from islets. These tumors are thought to develop from cells that are part of the diffuse neuroendocrine cell system (17-19).
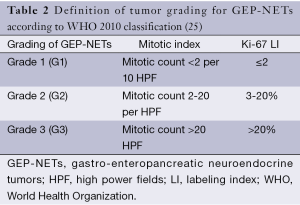
The World Health Organization (WHO) classifies GEP-NETs, according to their differentiation, histology, and measures of proliferation (including Ki-67). The WHO 2010 system describes all GEP-NETs as neoplasms with a malignant potential (20,21). Classification and grading of GEP-NETs according to WHO are presented in Tables 1,2.
Full table
GEP-NETs morphologically can be subdivided into well-differentiated NETs or poorly differentiated neuroendocrine carcinomas (NECs). Differentiation of the tumor is the main pathological variable determining patients’ prognosis and therapeutic approaches. NECs are high-grade (G3) malignant neoplasm. Macroscopically, an ill-defined solid mass with extensive necrosis is usual presentation of NECs. NECs resemble small cell carcinomas or large cell NECs of other organs because their cytology account for that of high-grade epithelial neoplasms. High mitotic activity and a proliferative rate higher than 20% are typical characteristics of NECs (25). Association with MEN1, size and site of tumor, metastases (particularly liver metastases), and histologic features are major prognostic factors (26).
Chromogranins, secretogranins, neuron-specific enolase, and subtilase preprotein convertases are markers used to identify neuroendocrine cells in the gastrointestinal system (1). General and tumor specific markers of GEP-NETs are presented in Table 3.
Somatostatin (SS) and SS receptors (sstrs) have been proven to control cell proliferation in GEP-NETs. Endothelial cells [via vascular endothelial growth factor (VEGF) and VEGF receptor (VEGFR)], and pericytes [via platelet-derived growth factor (PDGF) and the PDGF receptor (PDGFR)] are also crucial in encouraging angiogenesis and maintaining a continuous blood supply for proliferation of cancer cells. The PI3K-Akt and phospholipaseC/protein kinase C pathways are important for downstream VEGFR and PDGFR signaling. The PI3K/Akt/mTOR (mammalian target of rapamycin), RAS/RAF/MAPK, and JAK/STAT pathways are vital for signal transduction of IGF1-R and sstrs (28,29).
Clinical features and diagnosis
Since GEP-NETs are fairly rare, a high index of suspicion is needed to make diagnosis. Various peptides and neuroamines that might produce distinct syndromes are secreted by these tumors. Their diagnosis typically delayed by 5-7 years on average. As a result of this delay, the probability of metastatic disease is high (30,31).
Insulinoma
Insulinomas are endocrine tumors that originate in the pancreas, and excessive amounts of insulin are secreted by these tumors (7). Having an annual popularity incidence of 4 in every 1 million people, insulinoma is the most common NET of the pancreas and represent 1-2% of all pancreatic neoplasms. The average age at presentation is between 40 and 50 years, and 60% of the insulinomas occur among females in most series (32,33). Hypoglycemia secondary to the excess unregulated insulin secretion by the tumor is the clinical manifestation of insulinomas. Hypoglycemia is typically associated with fasting, and the hypoglycemic attacks characteristically occur before breakfast, but they may occur hours postprandial when a meal is delayed (7). Approximately 90% of insulinomas have been reported to be benign, more than 90% of them occur at intra-pancreatic sites, 90% are solitary, and 90% are lower than 2 cm in diameter. Most insulinomas exist in pancreas. Extra-pancreatic insulinomas causing hypoglycemia are rare, and their incidence is lower than 2%. Extra pancreatic insulinomas are mostly located in the duodenal wall (34). Insulinomas generally occur sporadically, but they can be associated with multiple endocrine neoplasia MEN1 syndrome (35).
Whipple and Frantz described the diagnostic triad for insulinoma in 1935:
- Symptoms of hypoglycemia provoked by fasting;
- Circulating glucose level less than 50 mg/dL under the presence of symptoms;
- Relief of symptoms with administration of glucose.
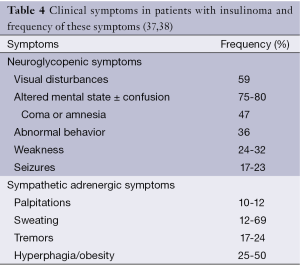
This is called as Whipple’s triad and also known as the diagnostic hallmark of insulinomas (36). Clinical symptoms in patients with insulinoma and frequency of these symptoms are listed in Table 4.
Full table
The biochemical diagnosis of insulinoma is established in 95% of patients during prolonged fasting (up to 72 hours) when the following results are found.
- Documentation of blood glucose level <50 mg/dL with hypoglycemic symptoms;
- Relief of symptoms after eating;
- Increased plasma insulin level (≥6 μU/mL);
- Increased C peptide level (≥0.2 nmol/L);
- Increased proinsulin level (≥5 pmol/L);
- Absence of plasma sulfonylurea.
That 72-hour fast in the hospital is usually applied to make the diagnosis. Plasma glucose, insulin, C-peptide, and proinsulin values should be measured at regular intervals (2-4 h) during this fasting period. Measurement should be done more frequently when blood sugar levels decrease to less than 50 mg/dL. Serum levels of the studies listed above should be repeated before administering glucose if the patient becomes symptomatic at any moment. When neuroglycopenic symptoms or persistent glucose levels of less than 40 mg/dL occur, the test must be terminated. About 70-80% of patients will develop hypoglycemia during the first 24 h of fasting, and 90-98% by 48 h. More than 90% of reported insulinomas have been detected in this manner. When blood glucose decreases to 40 mg/dL or less, serum insulin concentrations decrease to less than 6 μU/mL [i.e., assessed by the standard insulin radioimmunoassay (RIA)] in normal nonobese subjects and serum insulin to glucose ratio remains less than 0.3 (in mg/dL) (39-41). In at least 80-90% of patients with insulinoma, serum proinsulin has been notified to be increased (42).
Computed tomography and MRI can detect large tumors, but may cause false positives and false negatives. Only 50% to 70% of the insulinomas can be correctly localized by these techniques (1). If diagnosis of nesidioblastosis is suspected and reliable localization procedures are negative, 18F-DOPA positron emission tomography (PET) scans and the selective calcium infusion procedure will confirm the diagnosis of pancreatic hyperinsulinism as the probable cause of the hypoglycemia (43,44).
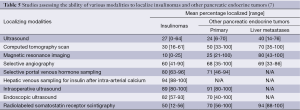
Endoscopic ultrasonography (EUS) is currently used as the test option for the localization of insulinomas in most of Western Centers. EUS is a highly reliable procedure for the preoperative localization of insulinomas, but there are several problems associated with using EUS for the detection of these tumors. First of all, EUS may yield both false-negative and false-positive results, and quality of the EUS findings are highly dependent on the examiner’s experience (45). Secondly, since insulinomas are completely isoechoic, some insulinomas may be overlooked by preoperative EUS. Female gender, a low body mass index and young age can increase the risk of negative imaging (46). As a final problem, localization and size of the tumor are important for the sensitivity of EUS in patients with insulinomas; sensitivity is the lowest for tumors in the tail of the pancreas or those that are extra-pancreatic and the greatest for those in the head of the pancreas (47). After the tumor site has been determined, preoperative diagnosis of insulinoma could be done by fine-needle aspiration (FNA) of the pancreas (48). Manual pancreas palpation done by an experienced surgeon and intraoperative ultrasonography (IOUS) are both sensitive methods for the intraoperative detection of the insulinoma site (49). Currently, preoperative transabdominal ultrasonography followed by IOUS is considered the most sensitive and the most specific approach which has been recommended for routine use. This approach along with palpation can be used to detect over 95% of tumors (50). The success rates of various modalities to localize insulinomas and other GEP-NETs are presented in Table 5.
Full table
Angiography combined with arterial stimulation venous sampling (ASVS) (angiography and ASVS) is a highly sensitive technique for the accurate localization of insulinomas, and usually provides more information than EUS. However, it should not precede CT or MRI (51). Morphological imaging modalities do not represent hormonal function, but ASVS helps localization of a tumor by affirming hormonal function. The use of ASVS can reduce the possibility of re-operation and allows for a more accurate surgical approach. In addition preoperative localization of atypical insulinomas by ASVS is especially important (52).
After diagnosis of an insulinoma, surgery is the first option for all localized tumors. The choice of process depends on the features of the tumor mass, such as type, size, and localization. Atypical resection such as enucleation, partial pancreatectomy, or middle pancreatectomy, has the advantage of protecting the pancreatic parenchyma as much as possible so that these types of resections decrease the risk of late exocrine/endocrine insufficiency (53). Most of benign insulinomas can be cured with surgery, although there are other techniques for the management of insulinomas such as octreotide injection, radiofrequency ablation (RFA), EUS guided alcohol ablation, or embolization (54-57).
As a SS analog, octreotide acts primarily via activation of SS sst2 receptors, and inhibits insulin secretion and the peripheral action of many gut hormones. Octreotide has been used for the treatment of insulinoma and successful control of blood glucose levels (58,59). Higher doses of standard SST analogs will possibly improve the clinical management of patients who fail to respond or cease to these drugs (60).
The percentages of reported incidence and the 10-year survival of malignant insulinomas are <10% and 29%, respectively (61,62). Liver and regional lymph nodes are the major sites of metastasis or recurrence. If improvement of patient survival is achievable, aggressive surgical resection such as extended pancreatic resection, liver resection and/or liver transplantation, RFA of neuroendocrine liver metastases should be attempted in patients with malignant insulinomas (63,64). In patients who have unresectable or uncontrollable malignant insulinomas, administration of octreotide and continuous glucose monitoring can be considered to both control hypoglycemic episodes and improve quality of life (65,66). The response of malignant insulinomas to chemotherapy is poor (1).
Glucagonoma
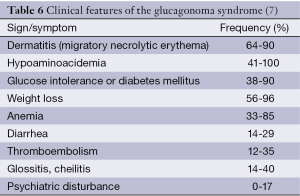
Glucagonomas originate mostly from pancreas. Besides, approximately 80% of glucagonomas generally occur sporadically, but they can be associated with multiple endocrine neoplasia MEN1 syndrome. At time of diagnosis, nearly 75% of glucagonomas are malignant and have metastasized. Necrolytic migratory erythema (NME), a rash that usually begins in the groin and perineum as a raised erythematous patch with occasional bullae, is the hallmark of this syndrome (8). Almost all of the glucagonoma patients have this characteristic lesion. Clinical features of the glucagonoma syndrome are presented in Table 6.
In 1966, the first well-described case of a patient with the glucagonoma syndrome was reported. The patient had elevated immunoreactive glucagon in plasma, diabetes mellitus, a skin rash, and a GEP-NETs (67). In a patient with chronic unexplained and therapy-resistant dermatitis and elevated erythrocyte sedimentation rates, glucagonoma should be suspected. Glucagonoma is associated with glucose intolerance and thromboembolic phenomena. Hepatitis B infections, short bowel syndrome, myelodysplastic disorders, malnutrition, celiac sprue, inflammatory bowel disease, cirrhosis, other malignancies, malabsorptive syndromes, and specific nutritional deficiencies can cause NME other than glucagonomas (7).
The plasma glucagon concentration is increased (>150 pg/mL) in almost all patients with glucagonoma (68,69). Familial hyperglucagonemia, renal insufficiency, severe stress (trauma, exercise, bacteremia, and diabetic ketoacidosis), acute pancreatitis, hepatic insufficiency, prolonged fasting, or other GEP-NETs can cause elevated plasma glucagon concentrations (7).
Ultrasound and CT scanning can identify the tumors since the majority of the tumors are large and metastatic. sstrs scintigraphy is the best method for describing the magnitude of metastatic disease. Localized solitary tumors should be excised surgically. In view of the thromboembolic complications, aspirin therapy is recommended and sometimes insulin is required for mild diabetes. SS analog therapy, hepatic artery embolization (HAE) of metastases, debulking surgery, and cytotoxic chemotherapy are palliative treatments for metastatic disease (1).
When SS analogues are used, NME is controlled in 50-90% of cases, and additionally abdominal pain, weight loss and diarrhea are usually improved. However, diabetes mellitus generally does not improve. Total parenteral nutrition (TPN) is frequently used in cases of cachexia. TPN also improves hypoaminoacidemia and leads to weight gain (70,71).
Gastrinoma
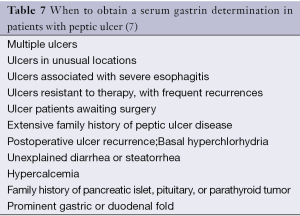
Although the adult pancreas do not normally express the gastrin gene, gastrinomas commonly originate from the pancreas and manifest as endocrine cell hyperplasia, endocrine carcinomas, solitary adenomas or microadenomas. Gastrinomas and insulinomas represent the two most common GEP-NETs (8). Gastrinomas are located primarily in the duodenum (60-80%) or pancreas (10-40%). Zollinger Ellison syndrome is a clinical syndrome due to the ectopic secretion of gastrin by a NET (gastrinoma) resulting in gastric acid hypersecretion. It can cause refractory peptic ulcer disease, severe gastroesophageal reflux disease, diarrhea and finally death, primarily due to the complications of the refractory peptic ulcer disease if left untreated (72). When to obtain a serum gastrin determination in patients with peptic ulcer is listed in Table 7. Approximately 75% of gastrinomas occur sporadically and about 25% are related to the MEN1 syndrome. Duodenal gastrinomas in patients with sporadic Zollinger-Ellison syndrome are frequently small and regional lymph node metastases are found in 60% of patients. Pituitary disease (60%), adrenal disease (45%), and carcinoid tumors (30%) can be found in patients presenting with gastrinoma as a component of the MEN1 syndrome (73,74).
Elevated fasting circulating gastrin levels (>200 pg/mL) and gastric acid hypersecretion (basal acid output >15 mEq/h with an intact stomach or >5 mEq/h after ulcer surgery) in patients off all acid antisecretory medication (14 days for proton pump inhibitors and 3 days for H2-receptor antagonists) are necessary for the diagnosis of gastrinoma. Serum gastrin values exceed 500 pg/mL in many patients with Zollinger-Ellison syndrome. If the serum gastrin levels are in the range from 200 to 500 pg/mL, a secretin stimulation test may be performed to confirm the diagnosis. Intravenous administration of secretin 0.4 mcg/kg over 1 minute (2 units/kg bolus) followed by serial measurement of serum gastrin levels at 2, 5, 10, 15, and 20 minutes is used as a provocative test. A doubling of the fasting gastrin level or an increase in the serum gastrin level of more than 200 pg/mL within 15 minutes strongly suggests the presence of a gastrinoma (8). Differential diagnosis of hypergastrinemia is presented in Table 8. Prominent gastric folds caused by the trophic effect of the chronic hypergastrinemia on the gastric mucosa are present in 92% of ZES patients (74).
Ninety percent of gastrinomas have been localized in the anatomical area known as the gastrinoma triangle. This is the area bounded by the junction of the second and third portions of the duodenum inferiorly, the confluence of the cystic and common bile ducts superiorly, and the junction of the neck and body of the pancreas medially. The duodenum is the most common extra-pancreatic site. Somatostatin receptor scintigraphy (SRS) is the first choice as a localization study, and majority of gastrinomas (more than 90%) will be detected combining it with CT and EUS (75). ASVS will provide both anatomical and functional information about the site of the gastrinoma and can be particularly useful for the difficult localization of a sporadic duodenal gastrinoma (76). Combining ASVS with conventional imaging studies contributes to increase in the rate of curative resection of gastrinoma (77).
In the past, only total gastrectomy was effective in controlling the gastric acid hypersecretion. However, with the development of histamine H2-receptor antagonists and later proton pumps inhibitors (PPIs), medical control is now possible in almost every patient, so that total gastrectomy is now rarely required (78). In the absence of unresectable disease, all patients with sporadic gastrinoma should undergo surgical exploration, which should include a combination of duodenal palpation, endoscopic transillumination, IOUS, and duodenotomy for the curative surgical resection. The primary tumor remains undetected at laparotomy despite precise exploration of the abdominal cavity in up to 20% of patients undergoing surgical exploration. In patients with gastrinoma and MEN1 syndrome, surgery usually is not indicated because these patients often have multiple, small pancreatic tumors that are not appropriate for surgical resection (8). Octreotide may have an antiproliferative effect, as well as a moderate antitumoral action, on progressive metastatic gastrinoma (79).
VIPoma
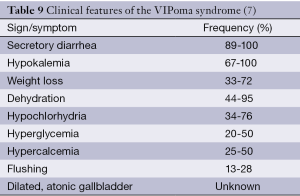
Profuse watery diarrhea, dehydration and hypokalemia with a non-β-cell pancreatic islet cell tumor, named as VIPoma syndrome was firstly described by Verner and Morrison in two patients. Therefore, VIPoma syndrome is also called as Verner-Morrison syndrome (80). Clinical features of the VIPoma syndrome are presented in Table 9.
VIPoma is an extremely rare NET that autonomously secretes VIP. The incidence of VIPoma is 1 per 10 million individuals per year (81). Only 6-11% of cases of VIPomas are associated with MEN1 syndrome, but VIPomas develop only in 1-3% of patients with MEN1 syndrome (7) VIPomas (90%) usually originate from pancreatic tissues (in adults) and the remaining 10% arise from extra-pancreatic tissues (in pediatrics) such as bronchus, liver, colon, and neural crest-derived tissues (sympathetic nerve chains, pituitary, thyroid and adrenal glands) (82).
Generally, the diagnosis of VIPoma is delayed. Majority of VIPomas (approximately 60-80%) have already been metastasized at the time of clinical diagnosis. Liver is the most common site of metastasis, however, lymph node, lung, and kidney metastases have also been notified (83-85).
Chronic, profuse, secretary, unaffected by fasting, exceeding 3 L/day, odorless, tea-colored, blood-free, mucus-free, high-sodium concentrated, and with low osmolal gap are the features of diarrhea that is found in VIPoma patients (84). Vasoactive intestinal peptide has been revealed as to cause net chloride secretion, to bind to specific receptors on intestinal epithelial cells, and to activate adenylate cyclase and increase level of cyclic AMP levels in intestinal cells (86). Peptide histidine isoleucine (PHI), another VIP-related peptide, is notified to be elevated in the serum of patients with VIPoma syndrome (87). The fasting plasma VIP concentration should be measured at the time when diarrhea is present, since VIP levels may be normal between diarrheal periods in an occasional patient with VIPoma. In one series of 29 patients with the VIPoma syndrome, the mean concentration of plasma VIP was 956 pg/mL, with a range of 225-1,850 pg/mL (88). Elevated levels of plasma VIP occasionally can be found in prolonged fasting, inflammatory bowel disease, small bowel resection, radiation enteritis, and chronic renal failure (89).
Ultrasonography, CT, SRS, or exploratory laparotomy with intraoperative ultrasound can be used for localization of VIPomas (8). Approximately 80-90% of VIPomas are sstrs-positive. Although being rarely used, octreoscan scintigraphy is an extremely helpful radiological intervention. Besides, IOUS is useful for the detection of obscure neoplasms (90,91).
The best chance of cure is surgical removal if possible. Surgical debulking of incurable disease is occasionally helpful in control of symptoms (1). In individuals, unwilling to undergo surgery or presenting with inoperable metastatic diseases, medical treatment is indicated. Octreotide has been shown to decrease VIP hormonal levels, control diarrhea greatly, and stabilize VIPoma tumor growth by its anti-proliferative properties (92).
Sunitinib can be a potential therapeutic option for VIPomas, because it decreases biochemical markers and stabilizes or reduces tumor bulk (93). Chemotherapy has been used for unresectable metastatic diseases, with varying success rates. The recommended combination regimen is doxorubicin/streptozocin. If doxorubicin is contraindicated, a 5-fluorouracil/streptozocin combination regimen may be considered (94).
VIPoma tumor grading, staging and surgical resectability are most important prognostic factors (95). The probability of 5-year survival is approximately 60% in patients with distant metastases (96).
Somatostatinoma
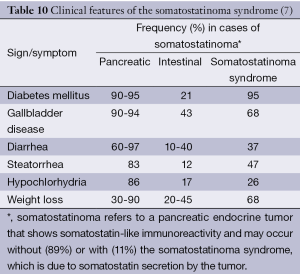
The first two cases of somatostatinomas were independently reported in 1977. Since then, only a handful of cases have been reported around the world. According to these reports, clinical somatostatinoma syndrome consisted of mild diabetes mellitus, anemia, weight loss and gallbladder disease. The incidence of somatostatinoma is extremely low as estimated to be 1 in 4 million (97-99). Other clinical features of somatostatinoma syndrome are described in Table 10.
Release of almost all other hormones is inhibited by SS. It has direct effects on a number of gastrointestinal functions such as potent inhibition of basal and pentagastrin or meal stimulated acid secretion, intestinal absorption of amino acids and cholecystokinin-stimulated pancreatic enzyme secretion (100,101). Somatostatinomas primarily occur in the pancreas (41-75%) or in the proximal small intestine (25-54%). Pancreatic somatostatinomas primarily occur in the pancreatic head (56-78%). Extra-pancreatic somatostatinomas occur in the duodenum (43-96%), ampullary area (48%), jejunum (5%), or cystic duct area. Almost all somatostatinomas (>96%) are solitary, and their diameter varies from less than 1 to 10 cm, with a mean of 4-5 cm. The mean age of patients with somatostatinomas is 45-50 years, with a range of 30-84 years (102-106).
Pancreatic tumors present late with hepatic metastases but local effects of duodenal tumors precede the development of the tumor syndrome, which is characterized by the triad of cholelithiasis, steatorrhea and diabetes (1). According to one large literature review of 173 cases of somatostatinomas, the somatostatinoma syndrome occurred in only 11% of patients (107).
Somatostatinomas are frequently later-stage malignancies with extensive liver metastases by the time of diagnosis, because many patients do not present with the classic triad and exhibit only nonspecific symptoms. Duodenal tumors are commonly associated with either neurofibromatosis type 1 or, less commonly, von Hippel-Lindau syndrome. They may also associate with pheochromocytomas (8). Inhibition of insulin and glucagon released by SS, and the replacement of functional islet tissue by tumor, cause the development of diabetes mellitus or glucose intolerance (103,107,108).
Tissue histopathology including special immunohistochemical staining is necessary for definite diagnosis. Surgery is the main treatment option for these patients. After surgery, adjuvant chemotherapy is not advocated. Although disease progression is slow, patients with somatostatinoma have an estimated 5-year survival rate of 60% to 100% after incomplete resection of the tumor (109).
Conventional and EUS, and CT can be used for localization of the duodenal tumors. Surgical resection can be curative in the small proportion of patients with localized disease. Chemotherapeutic agents such as streptozotocin and dacarbazine can be used in patients with incurable or recurrent disease (8). Because most somatostatinomas are located in the periampullary duodenum or pancreatic head, the most common surgery applied is the pylorus-preserving pancreaticoduodenectomy, but total pancreaticoduodenectomy can be necessary. Compared with alternative therapies such as HAE, RFA or radioactive octreotide, aggressive surgical resection of hepatic metastatic disease has been associated with improved outcome. Large size (>3 cm) of tumor, lymph node involvement and poor differentiation are negative prognostic factors. Non-functioning somatostatinomas predominate and, being typically poorly differentiated, have a worse prognosis than functioning somatostatinomas (109).
Carcinoid tumors
Carcinoids are uncommon NETs thought to originate from the enterochromaffin cells (Kulchitsky) cells present throughout the crypts of Lieberkühn of the gastrointestinal system (110). The 5-HT (serotonin), a vasoactive peptide which biosynthesis is accomplished particularly by the enterochromaffin cells, is the most common biologically active substance secreted from carcinoid tumors. The release of 5-HT, synthesized from the amino acid tryptophan, into the systemic circulation can cause the carcinoid syndrome whose classic symptoms include diarrhea, bronchoconstriction, episodic flushing, and eventual right-sided valvular heart disease (111).
Though relatively rare, carcinoid tumors represent the most common gastrointestinal NETs. Data derived from a 5-decade analysis of 13,715 carcinoid tumors revealed an overall increase in incidence over the past 30 years and the age-adjusted incidence rates for Caucasian men and women over the last decade were respectively 2.47 and 2.58 per 100,000 population per year. Regardless of the site of the tumor, a 5-year survival rate for patients is 67.2%. The location and stage of the tumor are factors affecting prognosis. The disease is considered to be more aggressive and with worse prognosis than what was thought in the past (112). The gastrointestinal tract (55%) and bronchopulmonary system (30%) are the major localizations of carcinoid tumors (113). Many carcinoid tumors are found during surgery for other reasons such as at appendectomy, surgery for acute pancreatitis and also surgery for bowel obstruction or diseases of the female reproductive tract (114,115).
Because symptoms usually are vague, nonspecific, and organ-related, the diagnosis is generally delayed. The mean time for onset of symptom to diagnosis is more than nine years. The mean age of patients with carcinoid tumor is 45-50 years (116). Measurement of serotonin metabolites in a 24-hour urine collection is mainstay for biochemical diagnosis of carcinoid tumors. Elevation of the 5-hydroxyindoleacetic acid (5-HIAA), pharmacologically inactive metabolite of serotonin, in a 24-hour sample has been reported to be highly specific (100%) for the diagnosis of carcinoid disease but this test has low sensitivity (73%) (117,118). In atypical carcinoid disease, 5-HIAA levels may not be elevated. Celiac sprue, Whipple’s disease, and small bowel obstruction can cause falsely elevated levels of 5-HIAA (119).
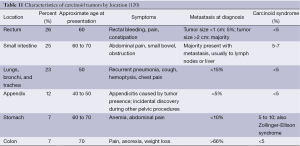
A carcinoid tumor often is only considered after the onset of carcinoid syndrome and which typically occur when the tumor has metastasized to the liver or lungs. Flushing (pale, purplish, or red), diarrhea (watery and explosive), bronchospasm, tachycardia or hypotension, telangiectasia, and right-side heart disease or failure is the symptoms of carcinoid syndrome. Characteristics of carcinoid tumors by location are presented in Table 11. Exertion, eating or drinking some foods [especially items high in tyramine (e.g., blue cheeses, chocolate) or ethanol (e.g., red wine)] are generally precipitating factors for symptoms (120-122).
For localization and staging of the disease, SRS and 131I- meta-iodobenzylguanidine (131I-MIBG) scanning have been used in recent years (123,124). Chest radiography, CT, or occasionally, bronchoscopy usually detects bronchial carcinoids (125). PET using 11 C-5-HTP, the precursor of serotonin synthesis, is a more sensitive method. This isotope accumulates in carcinoid tumors, and tumors as small as 0.5 cm in diameter can be detected with this technique (126). Carcinoid tumors also can be detected by [111In-DTPA-D-Phe1] octreotide scintigraphy (Octreoscan) with a sensitivity of 80% to 90% (127). 68Ga-DOTA-Tyr3-octreotide PET scanning has been reported to have a higher sensitivity than Octreoscan (128).
Symptomatic control and tumor reduction is mainstay of treatment of carcinoid tumors. Patients with carcinoid syndrome mostly have metastatic disease. Improvement of clinical symptoms, interruption of tumor growth, improvement of quality of life, and if possible, prolongation of overall survival (OS) are the therapeutic goals (129). Diuretics or angiotensin-converting enzyme (ACE) inhibitors can be required for heart failure due to carcinoid heart disease. Diarrhea in patients with carcinoid syndrome may be treated with loperamide or diphenoxylate (130). General treatment options for malignant carcinoid tumors are presented in Table 12. Octreotide or lanreotide can control clinical symptoms in about 60% to 70% of patients with carcinoid syndrome; these agents are considered the drugs of choice to control symptoms (131-133).
In addition to these syndromes, GEP-NETs secreting calcitonin cause a distinct syndrome with diarrhea; however, few cases have been described to establish them as constituting a distinct syndrome (134). Rare cases of GEP-NETs secrete renin and cause hypertension (135); others secrete erythropoietin, causing erythrocytosis and polycythemia (136). Similarly, the secretion of luteinizing-hormone causes masculinization (137,138), and the secretion of peptide YY (PYY) causes constipation (139).
Recent advances in diagnosis and treatment of GEP-NETs
Surgical resection is often curative, when GEP-NETs are diagnosed at an early stage. Unfortunately, curative surgery is rarely an option for patients with metastatic disease. The CT features of larger tumor size (>4.0 cm), transmural invasion, circumscribed tumor with both intra- and extra-luminal involvement, circumferential growth, mesenteric fat infiltration, ulceration, areas of cystic change or necrosis, and lymphadenopathy favor NECs over NETs. Tumor boundary, growth pattern, adjacent organ invasion, distant organ metastasis, degree of enhancement, and peritoneal seeding as a CT features do not distinguish between these two types of neuroendocrine neoplasms (140).
Compared with Octreoscan, MIBG scintigraphy and MRI, 68Ga-DOTATATE PET/CT has demonstrated superiority in lesion detection (141). 68Ga-labeled SS analogue PET/CT can influence many aspects of GEP-NETs management and has the potential to be the first-line imaging investigation for their evaluation (142).
mTOR pathway plays an important role in the pathogenesis of GEP-NETs. The mTOR inhibitor everolimus significantly delay disease progression in GEP-NETs patients. According to a randomized study of patients with advanced carcinoid tumors, the addition of everolimus to octreotide was associated with improved progression-free survival (PFS) compared with octreotide alone. But, the results were not satisfactory by means of the expected level in statistical significance. The results of studies examining everolimus in patients with advanced carcinoid tumors are awaited. Results of a randomized study evaluating the efficacy of everolimus in patients with nonfunctional gastrointestinal and lung NETs are anticipated. Results of ongoing and future studies will provide important information about the added benefit of combining everolimus with cytotoxic chemotherapy and other targeted agents, such as VEGF pathway inhibitors, in the treatment of advanced GEP-NETs (143). Higher severe toxicity occurred in everolimus treated patients who had previously been treated with systemic chemotherapy and peptide receptor radionuclide therapy (PRRT). This finding prompts caution while using everolimus in pretreated patients, and this drug may be used before PRRT and chemotherapy in the therapeutic algorithm for advanced GEP-NETs (144).
PRRT is a fresh and valuable treatment modality for patients with inoperable or metastasized GEP-NETs. PRRT is generally well tolerated and usually has mild and self-limiting acute side-effects. The objective response rates for PRRT in most studies are approximately 15-35%. PFS and OS in PRRT are more favorable than registered pharmaceutical and liver-directed therapies. Combining PRRT with radiosensitizing chemotherapeutical agents or combining PRRT with 90Y-octreotide or 177Lu-octreotate could improve the anti-tumoral efficacy. Intra-arterial administration of the radiopharmaceutical is an alternative way to improve uptake, in cases of high tumor load in the liver (145).
Surgery is the gold standard for curative therapy of GEP-NETs. If surgery is not suitable, like patients with liver metastases from GEP-NETs, minimally invasive therapeutic approaches can be applied. These therapeutic approaches are trans-arterial embolization (TAE), radiofrequency thermal ablation, trans-arterial chemoembolization (TACE) and new emerging techniques. The selective infusion of particles into the branch of the hepatic artery supplying the tumor lesions are cornerstone of TAE. The aim of TAE is to obstruct tumor blood vessels resulting in ischemia and necrosis. TAE is a safe therapeutic option in patients with liver metastases from GEP-NETs, control tumor progression, improve mass and endocrine symptoms and increase long term survival. TAE should be performed in an experienced GEP-NETs center, so as to minimize the risk related procedure (146).
According to the National Comprehensive Cancer Network (NCCN) guideline for NETs of the pancreas, TAE, TACE, radioembolization, cytoreductive surgery, ablative therapy, systemic chemotherapy and/or molecular-targeted therapies with everolimus or sunitinib are recommended as unresectable disease and/or distant metastases management (147). Chemotherapy, biotherapy with SS analogues/interferon-alpha, PRRT, molecular-targeted therapies, TAE and/or TACE is recommended by the European Neuroendocrine Tumor Society (ENETS) consensus guidelines for the management of unresectable liver metastases from digestive NETs (148).
Conclusions
The diagnosis of benign and incidentally identified lesions has increased due to the further availability of advanced endoscopic and radiological imaging. Consequently, the incidence and prevalence of GEP-NETs have increased substantially in past decades. A multidisciplinary approach of GEP-NETs provides best to achieve optimal clinical results. Specialists from different disciplines including, endocrinologists, gastroenterologists, oncologists, internists, radiologists, nuclear medicine specialists, surgeons and clinical geneticists should work actively together for the best results. According to the international guidelines (147,148), a multidisciplinary approach is currently advised for the optimal care of patients with a GEP-NET.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Taheri S, Ghatei MA, Bloom SR. Gastrointestinal Hormones And Tumor Syndromes. In: Jameson JL, De Groot LJ. eds. Endocrinology, Adult and pediatric. 6th ed. Philadelphia: Elsevier Saunders, 2010:2759-73.
- Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol 1902;28:325-53. [PubMed]
- Modlin IM, Kidd M. Ernest Starling and the discovery of secretin. J Clin Gastroenterol 2001;32:187-92. [PubMed]
- Rehfeld JF, Friis-Hansen L, Goetze JP, et al. The biology of cholecystokinin and gastrin peptides. Curr Top Med Chem 2007;7:1154-65. [PubMed]
- Dockray GJ, Varro A, Dimaline R, et al. The gastrins: their production and biological activities. Annu Rev Physiol 2001;63:119-39. [PubMed]
- Díez M, Teulé A, Salazar R. Gastroenteropancreatic neuroendocrine tumors: diagnosis and treatment. Ann Gastroenterol 2013;26:29-36. [PubMed]
- Jensen RT. Endocrine tumors of the pancreas. In: Yamada T, Alpers DH, Kaplowitz N, et al. eds. Textbook of Gastroenterology, 5th edn. Philadelphia: Lippincott Williams & Wilkins, 2009:1875-920.
- Vella A, Drucker DJ. Gastrointestinal hormones and gut endocrine tumors. In: Melmed S, Polonsky K, Larsen PR, et al. William’s textbook of endocrinology. 12th edn. Philadelphia: Saunders Elsevier, 2011:1697-716.
- Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61-72. [PubMed]
- Modlin IM, Shapiro MD, Kidd M. Siegfried Oberndorfer: origins and perspectives of carcinoid tumors. Hum Pathol 2004;35:1440-51. [PubMed]
- de Herder WW. Gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Best Pract Res Clin Gastroenterol 2012;26:689-90. [PubMed]
- Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol 2005;19:753-81. [PubMed]
- Fraenkel M, Kim MK, Faggiano A, et al. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol 2012;26:691-703. [PubMed]
- Eriksson B, Oberg K, Skogseid B. Neuroendocrine pancreatic tumors. Clinical findings in a prospective study of 84 patients. Acta Oncol 1989;28:373-7. [PubMed]
- Jensen RT, Norton JA. Endocrine neoplasms of the pancreas. In: Yamada T, Alpers DH. eds. Textbook of Gastroenterology, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 1999:2193.
- Jensen RT, Gardner JD. Gastrinoma. In: Go VLW, DiMagno EP. eds. The Pancreas: Biology, Pathobiology and Disease, 2nd ed. New York: Raven Press, 1993:931.
- Klöppel G. Tumour biology and histopathology of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab 2007;21:15-31. [PubMed]
- Perren A, Anlauf M, Henopp T, et al. Multiple endocrine neoplasia type 1 (MEN1): loss of one MEN1 allele in tumors and monohormonal endocrine cell clusters but not in islet hyperplasia of the pancreas. J Clin Endocrinol Metab 2007;92:1118-28. [PubMed]
- Langley K. The neuroendocrine concept today. Ann N Y Acad Sci 1994;733:1-17. [PubMed]
- Klimstra DS, Modlin IR, Adsay NV, et al. Pathology reporting of neuroendocrine tumors: application of the Delphic consensus process to the development of a minimum pathology data set. Am J Surg Pathol 2010;34:300-13. [PubMed]
- Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010;39:707-12. [PubMed]
- Williams ED, Siebenmann RE, Sobin LH, et al. eds. Histological typing of endocrine tumours. Geneva: World Health Organization; 1980.
- The International Agency for Research on Cancer, Hamilton SR, Aaltonen LA. eds. Pathology and genetics of tumours of the digestive system. Lyon, France: IARC Press, 2000.
- Bosman FT, Carneiro F, Hruban RH, et al. eds. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer, 2010.
- Capelli P, Fassan M, Scarpa A. Pathology - grading and staging of GEP-NETs. Best Pract Res Clin Gastroenterol 2012;26:705-17. [PubMed]
- Tomassetti P, Campana D, Piscitelli L, et al. Endocrine pancreatic tumors: factors correlated with survival. Ann Oncol 2005;16:1806-10. [PubMed]
- Kanakis G, Kaltsas G. Biochemical markers for gastroenteropancreatic neuroendocrine tumours (GEP-NETs). Best Pract Res Clin Gastroenterol 2012;26:791-802. [PubMed]
- Zarebczan B, Chen H. Signaling mechanisms in neuroendocrine tumors as targets for therapy. Endocrinol Metab Clin North Am 2010;39:801-10. [PubMed]
- Faivre S, Sablin MP, Dreyer C, et al. Novel anticancer agents in clinical trials for well-differentiated neuroendocrine tumors. Endocrinol Metab Clin North Am 2010;39:811-26. [PubMed]
- Modlin IM, Kidd M, Latich I, et al. Current status of gastrointestinal carcinoids. Gastroenterology 2005;128:1717-51. [PubMed]
- Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev 2004;25:458-511. [PubMed]
- Service FJ, McMahon MM, O’Brien PC, et al. Functioning insulinoma--incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc 1991;66:711-9. [PubMed]
- Grant CS. Insulinoma. Best Pract Res Clin Gastroenterol 2005;19:783-98. [PubMed]
- Okabayashi T, Shima Y, Sumiyoshi T, et al. Diagnosis and management of insulinoma. World J Gastroenterol 2013;19:829-37. [PubMed]
- Shin JJ, Gorden P, Libutti SK. Insulinoma: pathophysiology, localization and management. Future Oncol 2010;6:229-37. [PubMed]
- Whipple AO, Frantz VK. Adenoma of islet cells with hyperinsulinism: a review. Ann Surg 1935;101:1299-335. [PubMed]
- Boukhman MP, Karam JH, Shaver J, et al. Insulinoma--experience from 1950 to 1995. West J Med 1998;169:98-104. [PubMed]
- Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 2008;135:1469-92. [PubMed]
- Boden G. Glucagonomas and insulinomas. Gastroenterol Clin North Am 1989;18:831-45. [PubMed]
- Grunberger G, Weiner JL, Silverman R, et al. Factitious hypoglycemia due to surreptitious administration of insulin. Diagnosis, treatment, and long-term follow-up. Ann Intern Med 1988;108:252-7. [PubMed]
- Comi RJ, Gorden P, Doppman JL. Insulinoma. In: Go VLW, DiMagno EP. eds. The Pancreas: Biology, Pathobiology, and Disease, 2nd ed. New York: Raven Press, 1993:979.
- Vezzosi D, Bennet A, Fauvel J, et al. Insulin, C-peptide and proinsulin for the biochemical diagnosis of hypoglycaemia related to endogenous hyperinsulinism. Eur J Endocrinol 2007;157:75-83. [PubMed]
- Harrison TS, Fajans SS, Floyd JC Jr, et al. Prevalence of diffuse pancreatic beta islet cell disease with hyperinsulinism: problems in recognition and management. World J Surg 1984;8:583-9. [PubMed]
- Witteles RM, Straus FH II, Sugg SL, et al. Adult-onset nesidioblastosis causing hypoglycemia: an important clinical entity and continuing treatment dilemma. Arch Surg 2001;136:656-63. [PubMed]
- Kann PH, Rothmund M, Zielke A. Endoscopic ultrasound imaging of insulinomas: limitations and clinical relevance. Exp Clin Endocrinol Diabetes 2005;113:471-4. [PubMed]
- Kann PH, Ivan D, Pfützner A, et al. Preoperative diagnosis of insulinoma: low body mass index, young age, and female gender are associated with negative imaging by endoscopic ultrasound. Eur J Endocrinol 2007;157:209-13. [PubMed]
- Sotoudehmanesh R, Hedayat A, Shirazian N, et al. Endoscopic ultrasonography (EUS) in the localization of insulinoma. Endocrine 2007;31:238-41. [PubMed]
- Chang F, Chandra A, Culora G, et al. Cytologic diagnosis of pancreatic endocrine tumors by endoscopic ultrasound-guided fine-needle aspiration: a review. Diagn Cytopathol 2006;34:649-58. [PubMed]
- Wong M, Isa SH, Zahiah M, et al. Intraoperative ultrasound with palpation is still superior to intra-arterial calcium stimulation test in localising insulinoma. World J Surg 2007;31:586-92. [PubMed]
- Huai JC, Zhang W, Niu HO, et al. Localization and surgical treatment of pancreatic insulinomas guided by intraoperative ultrasound. Am J Surg 1998;175:18-21. [PubMed]
- Jackson JE. Angiography and arterial stimulation venous sampling in the localization of pancreatic neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab 2005;19:229-39. [PubMed]
- Guettier JM, Kam A, Chang R, et al. Localization of insulinomas to regions of the pancreas by intraarterial calcium stimulation: the NIH experience. J Clin Endocrinol Metab 2009;94:1074-80. [PubMed]
- Falconi M, Bettini R, Boninsegna L, et al. Surgical strategy in the treatment of pancreatic neuroendocrine tumors. JOP 2006;7:150-6. [PubMed]
- Rott G, Biggemann M, Pfohl M. Embolization of an insulinoma of the pancreas with trisacryl gelatin microspheres as definitive treatment. Cardiovasc Intervent Radiol 2008;31:659-62. [PubMed]
- Jürgensen C, Schuppan D, Neser F, et al. EUS-guided alcohol ablation of an insulinoma. Gastrointest Endosc 2006;63:1059-62. [PubMed]
- Limmer S, Huppert PE, Juette V, et al. Radiofrequency ablation of solitary pancreatic insulinoma in a patient with episodes of severe hypoglycemia. Eur J Gastroenterol Hepatol 2009;21:1097-101. [PubMed]
- Vezzosi D, Bennet A, Courbon F, et al. Short- and long-term somatostatin analogue treatment in patients with hypoglycaemia related to endogenous hyperinsulinism. Clin Endocrinol (Oxf) 2008;68:904-11. [PubMed]
- Kishikawa H, Okada Y, Hirose A, et al. Successful treatment of insulinoma by a single daily dose of octreotide in two elderly female patients. Endocr J 2006;53:79-85. [PubMed]
- Katabami T, Kato H, Shirai N, et al. Successful long-term treatment with once-daily injection of low-dose octreotide in an aged patient with insulinoma. Endocr J 2005;52:629-34. [PubMed]
- Baldelli R, Barnabei A, Rizza L, et al. Somatostatin analogs therapy in gastroenteropancreatic neuroendocrine tumors: current aspects and new perspectives. Front Endocrinol (Lausanne) 2014;5:7. [PubMed]
- Hirshberg B, Cochran C, Skarulis MC, et al. Malignant insulinoma: spectrum of unusual clinical features. Cancer 2005;104:264-72. [PubMed]
- Service FJ, McMahon MM, O’Brien PC, et al. Functioning insulinoma--incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc 1991;66:711-9. [PubMed]
- Gillams A, Cassoni A, Conway G, et al. Radiofrequency ablation of neuroendocrine liver metastases: the Middlesex experience. Abdom Imaging 2005;30:435-41. [PubMed]
- Olausson M, Friman S, Cahlin C, et al. Indications and results of liver transplantation in patients with neuroendocrine tumors. World J Surg 2002;26:998-1004. [PubMed]
- Sawyer AM, Schade DS. Use of a continuous glucose monitor in the management of inoperable metastatic insulinoma: a case report. Endocr Pract 2008;14:880-3. [PubMed]
- Okamoto M, Kishimoto M, Takahashi Y, et al. A case of malignant insulinoma: successful control of glycemic fluctuation by replacing octreotide injections with octreotide LAR injections. Endocr J 2013;60:951-7. [PubMed]
- McGavran MH, Unger RH, Recant L, et al. A glucagon-secreting alpha-cell carcinoma of the pancreas. N Engl J Med 1966;274:1408-13. [PubMed]
- Wermers RA, Fatourechi V, Kvols LK. Clinical spectrum of hyperglucagonemia associated with malignant neuroendocrine tumors. Mayo Clin Proc 1996;71:1030-8. [PubMed]
- Soga J, Yakuwa Y. Glucagonomas/diabetico-dermatogenic syndrome (DDS): a statistical evaluation of 407 reported cases. J Hepatobiliary Pancreat Surg 1998;5:312-9. [PubMed]
- Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 2008;135:1469-92. [PubMed]
- Jensen RT, Norton JA. Endocrine tumors of the Pancreas and Gastrointestinal Tract. In: Feldman M, Friedman LS, Brandt LJ. eds. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. Philadelphia: Saunders, 2010:491-522.
- Jensen RT, Niederle B, Mitry E, et al. Gastrinoma (duodenal and pancreatic). Neuroendocrinology 2006;84:173-82. [PubMed]
- Zogakis TG, Gibril F, Libutti SK, et al. Management and outcome of patients with sporadic gastrinoma arising in the duodenum. Ann Surg 2003;238:42-8. [PubMed]
- Gibril F, Schumann M, Pace A, et al. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore) 2004;83:43-83. [PubMed]
- Jensen RT, Cadiot G, Brandi ML, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology 2012;95:98-119. [PubMed]
- Doppman JL, Miller DL, Chang R, et al. Gastrinomas: localization by means of selective intraarterial injection of secretin. Radiology 1990;174:25-9. [PubMed]
- Shibata C, Funayama Y, Fukushima K, et al. Role of selective arterial secretin injection test in treatment of gastrinoma. Hepatogastroenterology 2006;53:960-3. [PubMed]
- Ito T, Igarashi H, Uehara H, et al. Pharmacotherapy of Zollinger-Ellison syndrome. Expert Opin Pharmacother 2013;14:307-21. [PubMed]
- Shojamanesh H, Gibril F, Louie A, et al. Prospective study of the antitumor efficacy of long-term octreotide treatment in patients with progressive metastatic gastrinoma. Cancer 2002;94:331-43. [PubMed]
- Verner JV, Morrison AB. Islet cell tumor and a syndrome of refractory watery diarrhea and hypokalemia. Am J Med 1958;25:374-80. [PubMed]
- Friesen SR. Update on the diagnosis and treatment of rare neuroendocrine tumors. Surg Clin North Am 1987;67:379-93. [PubMed]
- Krejs GJ. VIPoma syndrome. Am J Med 1987;82:37-48. [PubMed]
- Bieligk SC, Jaffe BM. VIPoma. In: Percopo V, Kaplan EL. eds. GEP and Multiple Neuroendocrine Tumors. Padua (Padova), Italy: Piccin Nuova Libraria SpA, 1996:357-69.
- Smith SL, Branton SA, Avino AJ, et al. Vasoactive intestinal polypeptide secreting islet cell tumors: a 15-year experience and review of the literature. Surgery 1998;124:1050-5. [PubMed]
- Ayub A, Zafar M, Abdulkareem A, et al. Primary hepatic vipoma. Am J Gastroenterol 1993;88:958-61. [PubMed]
- Dockray GJ. Vasoactive intestinal polypeptide and related peptides. In: Walsh JH, Dockray GJ. eds. Gut Peptides. New York: Raven Press, 1994:447.
- Bloom SR, Christofides ND, Delamarter J, et al. Diarrhoea in vipoma patients associated with cosecretion of a second active peptide (peptide histidine isoleucine) explained by single coding gene. Lancet 1983;2:1163-5. [PubMed]
- Park SK, O’Dorisio MS, O’Dorisio TM. Vasoactive intestinal polypeptide-secreting tumours: biology and therapy. Baillieres Clin Gastroenterol 1996;10:673-96. [PubMed]
- Schiller LR, Rivera LM, Santangelo WC, et al. Diagnostic value of fasting plasma peptide concentrations in patients with chronic diarrhea. Dig Dis Sci 1994;39:2216-22. [PubMed]
- Ghaferi AA, Chojnacki KA, Long WD, et al. Pancreatic VIPomas: subject review and one institutional experience. J Gastrointest Surg 2008;12:382-93. [PubMed]
- Aspestrand F, Kolmannskog F, Jacobsen M. CT. MR imaging and angiography in pancreatic apudomas. Acta Radiol 1993;34:468-73. [PubMed]
- Brentjens R, Saltz L. Islet cell tumors of the pancreas: the medical oncologist’s perspective. Surg Clin North Am 2001;81:527-42. [PubMed]
- Bourcier ME, Vinik AI. Sunitinib for the treatment of metastatic paraganglioma and vasoactive intestinal polypeptide-producing tumor (VIPoma). Pancreas 2013;42:348-52. [PubMed]
- Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med 1992;326:519-23. [PubMed]
- Karim N, Zarzour A, Daw HA, et al. Prolonged survival in a patient with metastatic vasoactive intestinal peptide producing pancreatic neuroendocrine tumors. J Clin Case Rep 2012;2:15.
- Soga J, Yakuwa Y. Vipoma/diarrheogenic syndrome: a statistical evaluation of 241 reported cases. J Exp Clin Cancer Res 1998;17:389-400. [PubMed]
- Ganda OP, Weir GC, Soeldner JS, et al. “Somatostatinoma”: a somatostatin-containing tumor of the endocrine pancreas. N Engl J Med 1977;296:963-7. [PubMed]
- Larsson LI, Hirsch MA, Holst JJ, et al. Pancreatic somatostatinoma. Clinical features and physiological implications. Lancet 1977;1:666-8. [PubMed]
- Zhang ZY, Zhang R, Wang L, et al. Diagnosis and treatment of pancreatic somatostatinoma: a case report. Chin Med J (Engl) 2008;121:2363-5. [PubMed]
- Jensen RT. Peptide therapy. Recent advances in the use of somatostatin and other peptide receptor agonists and antagonists. In: Lewis J, Lewis JH, Dubois A. eds. Current Clinical Topics in Gastrointestinal Pharmacology. Malden, MA: Blackwell Science, 1997:144.
- Guillermet-Guibert J, Lahlou H, Cordelier P, et al. Physiology of somatostatin receptors. J Endocrinol Invest 2005;28:5-9. [PubMed]
- Vinik AI, Strodel WE, Eckhauser FE, et al. Somatostatinomas, PPomas, neurotensinomas. Semin Oncol 1987;14:263-81. [PubMed]
- Sassolas G, Chayvialle JA. GRFomas, somatostatinomas: clinical presentation, diagnosis, and advances in management. In: Mignon M, Jensen RT. eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management. Series: Frontiers in Gastrointestinal Research. Basel: Karger, 1995:194-207.
- Soga J, Yakuwa Y. Somatostatinoma/inhibitory syndrome: a statistical evaluation of 173 reported cases as compared to other pancreatic endocrinomas. J Exp Clin Cancer Res 1999;18:13-22. [PubMed]
- Nesi G, Marcucci T, Rubio CA, et al. Somatostatinoma: clinico-pathological features of three cases and literature reviewed. J Gastroenterol Hepatol 2008;23:521-6. [PubMed]
- Moayedoddin B, Booya F, Wermers RA, et al. Spectrum of malignant somatostatin-producing neuroendocrine tumors. Endocr Pract 2006;12:394-400. [PubMed]
- Boden G, Shimoyama R. Somatostatinoma. In: Cohen S, Soloway RD. eds. Hormone-Producing Tumors of the Gastrointestinal Tract. New York: Churchill Livingstone, 1985:85.
- Vinik AI, Strodel WE, Eckhauser FE, et al. Somatostatinomas, PPomas, neurotensinomas. Semin Oncol 1987;14:263-81. [PubMed]
- Williamson JM, Thorn CC, Spalding D, et al. Pancreatic and peripancreatic somatostatinomas. Ann R Coll Surg Engl 2011;93:356-60. [PubMed]
- Pinchot SN, Holen K, Sippel RS, et al. Carcinoid tumors. Oncologist 2008;13:1255-69. [PubMed]
- Robiolio PA, Rigolin VH, Wilson JS, et al. Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation 1995;92:790-5. [PubMed]
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59. [PubMed]
- Maggard MA, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg 2004;240:117-22. [PubMed]
- Goede AC, Caplin ME, Winslet MC. Carcinoid tumour of the appendix. Br J Surg 2003;90:1317-22. [PubMed]
- Mayoral W, Salcedo J, Al-Kawas F. Ampullary carcinoid tumor presenting as acute pancreatitis in a patient with von Recklinghausen’s disease: case report and review of the literature. Endoscopy 2003;35:854-7. [PubMed]
- Horton KM, Kamel I, Hofmann L, et al. Carcinoid tumors of the small bowel: a multitechnique imaging approach. AJR Am J Roentgenol 2004;182:559-67. [PubMed]
- Sippel RS, Chen H. Carcinoid tumors. Surg Oncol Clin N Am 2006;15:463-78. [PubMed]
- Feldman JM, O’Dorisio TM. Role of neuropeptides and serotonin in the diagnosis of carcinoid tumors. Am J Med 1986;81:41-8. [PubMed]
- Robertson RG, Geiger WJ, Davis NB. Carcinoid tumors. Am Fam Physician 2006;74:429-34. [PubMed]
- de Vries H, Verschueren RC, Willemse PH, et al. Diagnostic, surgical and medical aspect of the midgut carcinoids. Cancer Treat Rev 2002;28:11-25. [PubMed]
- Oberg K, Astrup L, Eriksson B, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Part I-general overview. Acta Oncol 2004;43:617-25. [PubMed]
- Metcalfe DD. Differential diagnosis of the patient with unexplained flushing/anaphylaxis. Allergy Asthma Proc 2000;21:21-4. [PubMed]
- Krenning EP, Kwekkeboom DJ, Oei HY, et al. Somatostatin-receptor scintigraphy in gastroenteropancreatic tumors. An overview of European results. Ann N Y Acad Sci 1994;733:416-24. [PubMed]
- Taal BG, Hoefnagel CA, Valdés Olmos RA, et al. Combined diagnostic imaging with 131I-metaiodobenzylguanidine and 111In-pentetreotide in carcinoid tumours. Eur J Cancer 1996;32A:1924-32. [PubMed]
- Nessi R, Basso Ricci P, Basso Ricci S, et al. Bronchial carcinoid tumors: radiologic observations in 49 cases. J Thorac Imaging 1991;6:47-53. [PubMed]
- Orlefors H, Sundin A, Garske U, et al. Whole-body (11)C-5-hydroxytryptophan positron emission tomography as a universal imaging technique for neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and computed tomography. J Clin Endocrinol Metab 2005;90:3392-400. [PubMed]
- Janson ET, Westlin JE, Eriksson B, et al. [111In-DTPA-D-Phe1]octreotide scintigraphy in patients with carcinoid tumours: the predictive value for somatostatin analogue treatment. Eur J Endocrinol 1994;131:577-81. [PubMed]
- Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med 2007;48:508-18. [PubMed]
- Oberg K. Neuroendocrine Gastrointestinal and Lung Tumors (Carcinoid Tumors), Carcinoid Syndrome, and Related Disorders. In: Melmed S, Polonsky KS, Larsen PR, et al. eds. William’s textbook of endocrinology. 12th ed. Philadelphia: Elsevier Saunders, 2011:1809-28.
- Gregor M. Therapeutic principles in the management of metastasising carcinoid tumors: drugs for symptomatic treatment. Digestion 1994;55 Suppl 3:60-3. [PubMed]
- Lamberts SW, Krenning EP, Reubi JC. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev 1991;12:450-82. [PubMed]
- Scarpignato C. Somatostatin analogues in the management of endocrine tumors of the pancreas. In: Mignon M, Jensen RT. eds. Endocrine Tumors of the Pancreas. Basel: Karger, 1995:385-414.
- O’Toole D, Ducreux M, Bommelaer G, et al. Treatment of carcinoid syndrome: a prospective crossover evaluation of lanreotide versus octreotide in terms of efficacy, patient acceptability, and tolerance. Cancer 2000;88:770-6. [PubMed]
- Fleury A, Fléjou JF, Sauvanet A, et al. Calcitonin-secreting tumors of the pancreas: about six cases. Pancreas 1998;16:545-50. [PubMed]
- Langer P, Bartsch D, Gerdes B, et al. Renin producing neuroendocrine pancreatic carcinoma--a case report and review of the literature. Exp Clin Endocrinol Diabetes 2002;110:43-9. [PubMed]
- Samyn I, Fontaine C, Van Tussenbroek F, et al. Paraneoplastic syndromes in cancer: Case 1. Polycythemia as a result of ectopic erythropoietin production in metastatic pancreatic carcinoid tumor. J Clin Oncol 2004;22:2240-2. [PubMed]
- Piaditis G, Angellou A, Kontogeorgos G, et al. Ectopic bioactive luteinizing hormone secretion by a pancreatic endocrine tumor, manifested as luteinized granulosa-thecal cell tumor of the ovaries. J Clin Endocrinol Metab 2005;90:2097-103. [PubMed]
- Brignardello E, Manti R, Papotti M, et al. Ectopic secretion of LH by an endocrine pancreatic tumor. J Endocrinol Invest 2004;27:361-5. [PubMed]
- Kawano K, Ushijima K, Fujimoto T, et al. Peptide YY producing strumal carcinoid of the ovary as the cause of severe constipation with contralateral epithelial ovarian cancer. J Obstet Gynaecol Res 2007;33:392-6. [PubMed]
- Feng ST, Luo Y, Chan T, et al. CT Evaluation of Gastroenteric Neuroendocrine Tumors: Relationship Between CT Features and the Pathologic Classification. AJR Am J Roentgenol 2014;203:W260-6. [PubMed]
- Mojtahedi A, Thamake S, Tworowska I, et al. The value of (68)Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. Am J Nucl Med Mol Imaging 2014;4:426-34. [PubMed]
- Sharma P, Singh H, Bal C, et al. PET/CT imaging of neuroendocrine tumors with (68)Gallium-labeled somatostatin analogues: An overview and single institutional experience from India. Indian J Nucl Med 2014;29:2-12. [PubMed]
- Chan J, Kulke M. Targeting the mTOR Signaling Pathway in Neuroendocrine Tumors. Curr Treat Options Oncol 2014;15:365-79. [PubMed]
- Panzuto F, Rinzivillo M, Fazio N, et al. Real-world study of everolimus in advanced progressive neuroendocrine tumors. Oncologist 2014;19:966-74. [PubMed]
- van der Zwan WA, Bodei L, Mueller-Brand J, et al. GEP-NETS update: Radionuclide therapy in neuroendocrine tumors. Eur J Endocrinol 2014. [Epub ahead of print]. [PubMed]
- Del Prete M, Fiore F, Modica R, et al. Hepatic arterial embolization in patients with neuroendocrine tumors. J Exp Clin Cancer Res 2014;33:43. [PubMed]
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Version 1.2012 Neuroendocrine tumors of the pancreas. Available online: http://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012;95:157-76. [PubMed]