P-glycoprotein plays an important role in the cross-resistance to taxanes in 5FU-resistant gastric cancer cells
Introduction
In spite of recent advances in the development of adjuvant therapy (1) and diagnostic tools, recurrent tumors are often detected even after curative surgery, and many patients with gastric cancer are still diagnosed only at the late stages after surgery is no longer curative. Although the median survival (MST) of metastatic gastric cancer improved from 3-5 to 13 months following the development of new chemotherapeutic agents including S-1, taxanes, CPT-11 and platinum derivatives (2-5), gastric cancer remains one of the major causes of cancer death worldwide (6). Most patients with advanced and metastatic gastric cancer are treated with multiple-line chemotherapies, and sometimes, second-line chemotherapy is not as effective as expected even when the second-line agents have different mechanisms of action from the first-line agents (7,8).
Many different chemotherapeutic regimens used for gastric cancer and gastrointestinal malignancies include 5FU (1,2,9). The first step in the activation of 5FU is the phosphorylation of 5FU by orotate phosphoribosyltransferase (OPRT), which metabolizes 5FU to 5-fluorouridine monophosphate (FUMP) in the presence of 5-phosphoribosyl 1-pyrophosphate. Then, 5FU is finally metabolized to its active metabolite, 5-fluorodeoxyuridine diphosphate (FdUMP), and it forms a covalent ternary complex with the DNA de novo synthesizing enzyme, thymidine synthetase (TS), together with the coenzyme, 5,10-methylenethtrahydrofolate (MTHF). This complex blocks the conversion of deoxyuridine monophosphate (dUMP) to thymidine monophosphate (dTMP) and thus inhibits DNA synthesis. 5FU is catabolized to 2-fluorob-alanin by dihydropyrimidine dehydrogenase (DPD) (10). Therefore, the enzymes involved in the metabolism of 5FU, such as OPRT, TS and DPD, could be predictive markers for the response to 5FU (10).
Taxanes, such as docetaxel and paclitaxel, are novel chemotherapeutic agents that promote the assembly of microtubules from tubulin dimers, and inhibit the depolymerization of tubulin, which stabilize microtubules in the cell. This results in the inhibition of DNA, RNA and protein synthesis (11). Docetaxel and paclitaxel have shown promising activity in gastric cancer, both as monotherapy (12) and in combination with other agents (3,4,13).
MKN45/F2R cells with reduced OPRT gene expression in comparison to MKN45 parent cells were previously established in order to elucidate the mechanism of 5FU resistance (14). Interestingly, the MKN45/F2R cells also showed resistance to docetaxel and paclitaxel, as well as other chemotherapeutic agents.
It is important to understand the mechanism of cross-resistances for more efficacious treatment. The present study was performed to clarify the mechanism of cross-resistance between 5FU and taxanes in 5FU-resistant cells, and also discusses a novel strategy to overcome such cross-resistance.
Materials and methods
Drugs
The 5FU, paclitaxel, docetaxel were kindly provided by Kyowa Hakko (Tokyo, Japan), Bristol-Myers Squibb (Tokyo, Japan), Sanofi Aventis (Tokyo, Japan), respectively. Verapamil was purchased from Wako (Osaka, Japan).
Cell lines and cell culture
MKN45 cells are poorly differentiated human gastric adenocarcinoma cells. The MKN45 cells were cultured in RPMI 1640 medium (Wako) supplemented with 10% fetal bovine serum (Sigma-Aldrich, St., Louis, MO, USA), antibiotics (Sigma-Aldrich), and HEPES (Sigma-Aldrich) in a humidified atmosphere of 5% CO2 at 37 °C. MKN45/F2R cells are a 5FU-resistant cell line. This line was established by continuously exposing the MKN45 parent cells to increasing concentrations (0.1-2 µM) of 5FU over the course of a year. The MKN45/F2R cells were routinely maintained in culture medium containing 2 µM of 5FU. The resistant cells were cultured in drug-free medium for at least 2 weeks before all of the studies to eliminate the effects of 5FU in the experiments (14).
Western blot analysis and antibodies
The cells were harvested and lysed in RIPA buffer (Sigma-Aldrich) for 15 minutes on ice. The protein concentration of the lysates was measured using a DC Protein Assay Kit (Bio-Rad, Hercules, CA, USA). The cell lysates were boiled in Sample Buffer Solution (Wako). Total cell protein extracts (20 µg/lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using SuperSepTM (Wako), and were electrophoretically transferred onto polyvinyl difluoride membranes. The membranes were blocked with PVDF blocking reagent (TOYOBO, Osaka, Japan) for 1 h. The membranes were then incubated with primary antibodies against β-actin, DPD (purchased from Cell Signaling Technology 1:5,000), TS and OPRT (kindly provided by Taiho Pharmaceutical Company, Tokyo, Japan 1:10,000) overnight at 4 °C. The primary antibodies were diluted with Can Get Signal Solution 1 (TOYOBO). The membranes were then washed with Dako Washing Buffer (Dako, Denmark) and incubated with the appropriate secondary antibodies (Millipore 1:25,000). The secondary antibodies were diluted with Can Get Signal Solution 2 (TOYOBO). The immunoreactive proteins were visualized by chemiluminescence using ImmunoStar LD reagents (Wako), and images were captured by a LAS-4000 device (FUJIFILM, Tokyo, Japan).
The 3-(4,5-dimethyl-2-tetrazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT) assay for the effects of 5FU or oxaliplatin
Cell growth was assessed by a standard 3-(4,5-dimethyl-2-tetrazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT) assay, which detects the dehydrogenase activity in viable cells. A total of 5×103 cells were seeded onto each well of 96-well culture plates, and were cultured for 24 h. The cells were treated with various concentrations of drugs for 72 h, the culture medium was removed, and 100 µL of a 0.5 mg/mL solution of MTT (Sigma-Aldrich) was added to each well. The plates were then incubated for 4 h at 37 °C. The culture medium was replaced with 100 µL of dimethyl sulfoxide (Wako) per well, and the absorbance at 540 nm was measured using an Envision 2104 Multilabel Reader (Perkin Elmer, Waltham, MA, USA). Each assay was repeated three times, and the mean half maximal inhibitory concentration (IC50) values were calculated based on the results of the MTT assay. The significance of differences in IC50s was tested using Student’s t-test.
Transfection and small interfering RNA experiments for OPRT
The MKN45 cells were cultured in medium without antibiotics for 24 h to 50-70% confluence before transfection. The cells were transfected with a small interfering RNA (siRNA) oligonucleotide using Lipofectamine RNAiMAX (Invitrogen) in a final siRNA concentration of 40 nmol/L in serum-free Opti-MEM (Invitrogen) for 48 h. The total RNA and proteins were extracted, and the expression levels of the OPRT mRNA and protein were analyzed by real-time RT-PCR and a Western blotting analysis, respectively. The siRNA oligonucleotides for OPRT (Stealth RNAi) and the negative control oligonucleotides (Stealth RNAi siRNA Negative Control) were purchased from Invitrogen.
Results
Changes in the expression levels of the TS, DPD and OPRT
The cells used in the present study were parental MKN45 cells and 5FU-resistant MKN45/F2R cells that were previously established as 5FU-resistant cells. MKN45/F2R cells showed 78.3-fold increased resistance to 5FU in comparison to the parental MKN45 cells. The MKN45/F2R cells also showed resistance to paclitaxel and docetaxel. The major characteristics of these cell lines were consistent with those reported previously (14).
TS, DPD and OPRT play key roles in the functions of 5FU. Therefore, the expression of the proteins was examined to understand the mechanism of acquired resistance in the MKN45/F2R cells.
Both cell lines were first treated with 1, 10 and 100 µM 5FU for 24 h, and the expression levels of the TS, DPD and OPRT proteins were investigated by a Western blot analysis. Figure 1A shows that the expression of TS was detected as double bands. The lower band (smaller molecular weight) was considered to be free TS (36kD), and the upper band (larger molecular weight) was considered to be the ternary complex formed by TS, FdUMP and MTHF. The ternary complex in the parental MKN45 cells emerged when they were treated with 1 µM 5FU. The band for the ternary complex was markedly reduced by treatment with 1 and 10 µM in the 5FU-resistant MKN45/F2R cells. The ternary complex emerged in both cell types following treatment with 100 µM of 5FU (Figure 1A). Both cell types were then treated with 10 µM of 5FU for 3, 6, 12, 24 and 48 h. The ternary complex in MKN45 cells emerged after 3 h, while it emerged only after 24 h in the MKN45/F2R cells (Figure 1B).
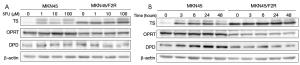
The expression of OPRT was decreased to 42% in the MKN45/F2R cell line in comparison to the MKN45 parental cell line, and was not altered by treatment with 5FU in either cell type (Figure 1A,B). DPD was detected in both cell lines, and there was no significant difference in the expression between the two cell lines (Figure 1A,B).
These results indicated that the decreased expression of OPRT led to reduced formation of the TS ternary complex. Therefore, decreased OPRT may be one of the causes of 5FU resistance in MKN45/F2R cells.
Increased sensitivity to 5FU, and unchanged sensitivity to taxanes, after transfection of a siRNA against OPRT in MKN45 cells
A siRNA against OPRT was transfected into the MKN45 parental cells, and the sensitivity was analyzed to confirm whether the decreased expression of OPRT directly induced resistance to 5FU or taxanes. The mRNA and protein expression levels of OPRT in the transfected cells were investigated before analyzing the IC50, and they were found to be markedly decreased to 10.3% and 50.0%, respectively, in untreated cells (Figure 2A,B). Next, the IC50 values for 5FU, paclitaxel and docetaxel were examined by using an MTT assay. The IC50 values for 5FU in the MKN45 cells transfected with siRNA increased to 65.6 µM, thus 62.5-fold resistance was obtained after transfection (Figure 2C, Table 1). Meanwhile, the resistance to paclitaxel and docetaxel in the MKN45 cells was not altered after siRNA transfection (Figure 2D,E). The IC50 values under these conditions are shown in Table 1.
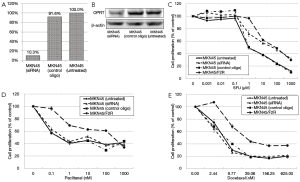

Full table
Increased p-glycoprotein expression contributes to cross-resistance to taxanes in 5FU-resistant MKN45/F2R cells
The expression of several molecules was examined using a Western blot analysis to elucidate the mechanism underlying the cross-resistance to taxanes in the MKN45/F2R cells. The expression levels of p-glycoprotein, β-III tubulin and Bcl-2, which are involved in taxane resistance, were examined in the parental MKN45 cells and 5FU-resistant MKN45/F2R cells. The expression of p-glycoprotein was increased 4.5-fold in the MKN45/F2R cells in comparison to the MKN45 parental cells (Figure 3A). P-glycoprotein is an efflux pump for taxanes and other drugs, such as doxorubicin (15), and it can be competitively inhibited by verapamil (16). The sensitivity of the cells to 5FU and taxanes was analyzed in the presence and absence of verapamil to confirm whether p-glycoprotein contributes to the resistance to 5FU or taxanes.
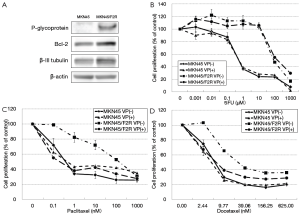
The cells were cultured in media containing various concentrations of paclitaxel, docetaxel or 5FU with or without 10 µM of verapamil, and the cell viability was measured using the MTT assay. Figure 3B shows that the resistance to 5FU in the MKN45/F2R cells was not reversed when they were cultured with verapamil. Meanwhile, the resistance to paclitaxel and docetaxel was reversed to the level of the parental MKN45 cells when they were cultured with verapamil (Figure 3C,D). The IC50 values under these conditions are shown in Table 2. These results indicated that p-glycoprotein played a principal role in the cross-resistance to taxanes, but it did not contribute to 5FU resistance in MKN45/F2R cells.
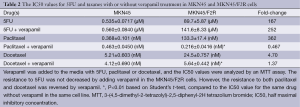
Full table
Discussion
The roles of TS, DPD and OPRT in 5FU metabolism have been studied by many researchers. OPRT has received extensive attention because its expression is believed to be correlated with the sensitivity to 5FU (17,18). The sensitivity to 5FU was increased in OPRT transfected cells in vitro and in vivo (19). The 5FU-resistant MKN45/F2R cells were established from MKN45 gastric cancer cells to study the mechanism underlying drug resistance in gastric cancer (14). The MKN45/F2R cells showed 78.3-fold increased resistance to 5FU compared to the parental cells, and the expression of OPRT was decreased in these cells. Furthermore, OPRT-knockdown MKN45 cells showed resistance to 5FU (62.5-fold) in comparison to the control cells. These results strongly indicated that decreased OPRT expression plays an important role in the resistance to 5FU in gastric cancer cells.
Tumor cells that are resistant to one anticancer drug often acquire resistance to other drugs, and the MKN45/F2R cells also showed cross-resistance to paclitaxel and docetaxel, as well as other drugs. P-glycoprotein, β3 tubulin and Bcl-2 are associated with resistance to taxanes (20-22), and the expression of p-glycoprotein in the 5FU-resistant MKN45/F2R cells was markedly increased in comparison to parental MKN45 cells. P-glycoprotein is an ATP-dependent drug efflux pump for drugs such as taxanes and doxorubicin (15), and it can be competitively inhibited by verapamil (16). The resistance to docetaxel and paclitaxel in MKN45/F2R were reversed in the presence of verapamil, suggesting that the increased expression of p-glycoprotein was the main cause of the taxane resistances in 5FU-resistant MKN45/F2R cells.
These results raised another question. P-glycoprotein can transport various hydrophobic agents, such as taxanes and doxorubicin, but 5FU is hydrophilic, and cannot be transported by p-glycoprotein (23). The resistance to 5FU in MKN45/F2R cells was not reversed in the presence of verapamil. Therefore, it is unclear why 5FU-resistant MKN45/F2R cells expressed p-glycoprotein.
However, these results do offer a suggestion. Constant exposure to 5FU may lead to elevated expression of p-glycoprotein, causing cancer cells develop cross-resistance to taxanes (24). Some researchers have reported that 5FU is still effective even when increased expression of p-glycoprotein was recognized in tumor cells (23,25,26). These findings may suggest that taxanes should be used prior to 5FU. This is contradicts the standard strategy for first-line therapy. The exposure to taxanes may lead to elevated expression of p-glycoprotein in cancer cells, but 5FU may still be effective even after treatment with taxanes because it is not subject to p-glycoprotein-mediated transport (23,25). Although the present study did not clarify whether exposure to taxanes induced the expression of p-glycoprotein, but this has been noted in other studies (23,25).
In conclusion, the current study revealed that p-glycoprotein plays a principal role in the cross-resistance to taxanes in 5FU-resistant gastric cancer cells, and suggests that treatment with taxanes prior to 5FU should be considered for patients with gastric cancer.
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (C) from the Ministry of Education, Science, Sports, and Culture of Japan.
Authors’ Contributions: R Mori designed the overall study, carried out experiments, collected and analyzed data, and wrote the paper. K Yoshida supervised this study, designed experiments and edited the paper. N Okumura, K Yamaguchi, and M Futamura advised R Mori on interpretation of data, and reviewed the paper. T Tanahashi, K Yawata, and J Kato designed and carried out the experiments. All authors read and approved the final manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Tsuburaya A, Yoshida K, Kobayashi M, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol 2014;15:886-93. [PubMed]
- Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215-21. [PubMed]
- Yoshida K, Ninomiya M, Takakura N, et al. Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res 2006;12:3402-7. [PubMed]
- Koizumi W, Kim YH, Fujii M, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol 2014;140:319-28. [PubMed]
- Mori R, Yoshida K, Tanahashi T, et al. Decreased FANCJ caused by 5FU contributes to the increased sensitivity to oxaliplatin in gastric cancer cells. Gastric Cancer 2013;16:345-54. [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Yoshida T, Yoshikawa T, Tsuburaya A, et al. Feasibility study of biweekly CPT-11 plus CDDP for S-1- and paclitaxel-refractory, metastatic gastric cancer. Anticancer Res 2006;26:1595-8. [PubMed]
- Hironaka S, Zenda S, Boku N, et al. Weekly paclitaxel as second-line chemotherapy for advanced or recurrent gastric cancer. Gastric Cancer 2006;9:14-8. [PubMed]
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37. [PubMed]
- Maring JG, Groen HJ, Wachters FM, et al. Genetic factors influencing pyrimidine-antagonist chemotherapy. Pharmacogenomics J 2005;5:226-43. [PubMed]
- Dumontet C, Sikic BI. Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J Clin Oncol 1999;17:1061-70. [PubMed]
- Bang YJ, Kang WK, Kang YK, et al. Docetaxel 75 mg/m(2) is active and well tolerated in patients with metastatic or recurrent gastric cancer: a phase II trial. Jpn J Clin Oncol 2002;32:248-54. [PubMed]
- Kornek GV, Raderer M, Schüll B, et al. Effective combination chemotherapy with paclitaxel and cisplatin with or without human granulocyte colony-stimulating factor and/or erythropoietin in patients with advanced gastric cancer. Br J Cancer 2002;86:1858-63. [PubMed]
- Tsutani Y, Yoshida K, Sanada Y, et al. Decreased orotate phosphoribosyltransferase activity produces 5-fluorouracil resistance in a human gastric cancer cell line. Oncol Rep 2008;20:1545-51. [PubMed]
- Leslie EM, Deeley RG, Cole SP, et al. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 2005;204:216-37. [PubMed]
- McDevitt CA, Callaghan R. How can we best use structural information on P-glycoprotein to design inhibitors? Pharmacol Ther 2007;113:429-41. [PubMed]
- Fujii R, Seshimo A, Kameoka S, et al. Relationships between the expression of thymidylate synthase, dihydropyrimidine dehydrogenase, and orotate phosphoribosyltransferase and cell proliferative activity and 5-fluorouracil sensitivity in colorectal carcinoma. Int J Clin Oncol 2003;8:72-8. [PubMed]
- Kodera Y, Ito S, Fujiwara M, et al. Gene expression of 5-fluorouracil metabolic enzymes in primary gastric cancer: correlation with drug sensitivity against 5-fluorouracil. Cancer Lett 2007;252:307-13. [PubMed]
- Taomoto J, Yoshida K, Wada Y, et al. Overexpression of the orotate phosphoribosyl-transferase gene enhances the effect of 5-fluorouracil on gastric cancer cell lines. Oncology 2006;70:458-64. [PubMed]
- Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol 2007;18 Suppl 5:v3-8. [PubMed]
- Mozzetti S, Ferlini C, Concolino P, et al. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res 2005;11:298-305. [PubMed]
- Yoshino T, Shiina H, Urakami S, et al. Bcl-2 expression as a predictive marker of hormone-refractory prostate cancer treated with taxane-based chemotherapy. Clin Cancer Res 2006;12:6116-24. [PubMed]
- Liu B, Staren ED, Iwamura T, et al. Mechanisms of taxotere-related drug resistance in pancreatic carcinoma. J Surg Res 2001;99:179-86. [PubMed]
- Takechi T, Koizumi K, Tsujimoto H, et al. Screening of differentially expressed genes in 5-fluorouracil-resistant human gastrointestinal tumor cells. Jpn J Cancer Res 2001;92:696-703. [PubMed]
- Breen L, Murphy L, Keenan J, et al. Development of taxane resistance in a panel of human lung cancer cell lines. Toxicol In Vitro 2008;22:1234-41. [PubMed]
- Savas B, Arslan G, Gelen T, et al. Multidrug resistant malignant melanoma with intracranial metastasis responding to immunotherapy. Anticancer Res 1999;19:4413-20. [PubMed]


