Implications of epithelial-mesenchymal transition in gastric cancer
Introduction
Gastric cancer has a variety of phenotypes (1). One of its interesting features is the differences between diffuse- and intestinal-type gastric cancers. The epithelial-mesenchymal transition (EMT) might explain these phenotypic differences. Cadherin 1, type 1, E-cadherin (epithelial) (CDH1) is commonly up-regulated in intestinal-type gastric cancer. In recent studies, mutations or gene alterations in CDH1 have been associated with gastric cancer malignancy or metastatic ability. In this review, we describe the biological roles of CDH1 in gastric cancer association with EMT. Gene expression profiling of gastric cancer has revealed that both cancer grades and stages can be identified via gene signatures (2). In addition, gene and genome alterations have been examined to detect cell phenotypes. Based on a comprehensive analysis of the gastric cancer genome, a number of genes, including CDH1, tumor protein p53 (TP53), AT rich interactive domain 1A (SWI-like) (ARID1A), mucin 6, oligomeric mucus/gel-forming (MUC6), catenin (cadherin-associated protein), alpha 2 (CTNNA2), GLI family zinc finger 3 (GLI3), and ring finger protein 43 (RNF43) have been identified as mutated driver genes (3). These findings suggest the significance of molecular information in cancer prognosis and treatment.
In several diseases, the expression of EMT-related genes, including CDH1, has been demonstrated to be negatively regulated (4-8). However, the loss of CDH1 is insufficient to induce EMT, suggesting that combinations of genes are involved in the EMT process (9). In this review, we focus on the biological roles of CDH1 in gastric cancer and discuss the cellular phenotypic alterations.
CDH1 and gastric cancer
CDH1 is one of the frequently mutated driver genes in gastric cancer, particularly in the diffuse-type gastric cancers (3,10-13). Generally, CDH1 is up-regulated in intestinal-type gastric cancer and down-regulated in diffuse-type gastric cancers, whereas cadherin 2, type 1, N-cadherin (neuronal) (CDH2) is up-regulated in the diffuse-type gastric cancer (14). Analyses of CDH1- and TP53-mutated gastric cancers suggest that transforming growth factor-beta receptor 2 (TGFBR2) is a candidate driver gene that plays a role as a metastasis suppressor (7). Germline mutations in CDH1 have been associated with human hereditary diffuse gastric carcinoma (15,16). Analyses using the Catalogue of somatic mutations in cancer (COSMIC) database (http://www.sanger.ac.uk/genetics/CGP/cosmic/) have revealed that CDH1 mutations are also associated with diffuse-type gastric cancer (17). Whereas CDH1 is mutated in approximately 40% of gastric cancer cases, germline mutations in mitogen-activated protein kinase kinase kinase 6 (MAP3K6) have been associated with gastric cancers without CDH1 mutations (5). The -160C to a promoter polymorphism and haplotypes of CDH1 have been associated with the risk of developing sporadic diffuse-type gastric cancer (18).
A previous study has shown that CDH1 expression was increased in gastric cancer cells co-expressing a putative mitogen-activated protein kinase activator with WD40 repeats (MAWD) and a MAWD binding protein (MAWBP), and they were treated with TGF-1 (19). CDH1, SMAD family member 4 (Smad4) and p53 play important roles in gastric cancer formation (20). The loss of CDH1 and Smad4 expression promotes diffuse-type gastric adenocarcinoma and metastasis (20).
Gastrokine 1, a molecule associated with gastric mucosal defense, is reduced in 36.4% of gastric mucosal tissues and is related to miR-185 expression (21). Considering that the Gastrokine 1-miR-185-DNA methyltransferase (DNMT) 1 axis is suggested as a suppressor of gastric carcinogenesis, the influence of gastrokine-regulated methylation on tumor progression should be investigated (21). Indeed, CDH1 methylation was detected in more than 80% of gastric mucosal tissues examined in this study (21). CDH1, claudin-10 and claudin-17 are down-regulated in gastric cancer (22). The down-regulation of CDH1 might be involved in cancer promotion. Germline variants of CDH1 have been identified in sporadic gastric cancer patients, and the involvement of down-regulation in CDH1 is indicated (23). In gastric cancer, CDH1 is also regulated through cyclooxygenase-2 (COX-2) via the nuclear factor (NF)-κB pathway (24). Several somatic mutations of genes, including erb-b2 receptor tyrosine kinase 2 (ERBB2) (HER2) and CDH1 have been detected in gastric cancer (25). Diffuse-type gastric cancer might arise from the down-regulation of CDH1 (25). However, the expression of ERBB2 is preferentially up-regulated in intestinal-type gastric cancers, and the prognostic value of ERBB2 in gastric cancer remains controversial (25,26). The methylation status of CDH1 is altered through Helicobacter pylori (H. pylori) infection (27-29). CDH1 expression at the plasma membrane is decreased in gastroesophageal junction adenocarcinoma associated with metastasis (30). The metastasis-associated gene (MTA3) is also decreased in tumor tissues, suggesting that the EMT pathway is regulated via MTA3, a potential prognostic factor in gastroesophageal junction adenocarcinoma (30). Aquaporin 3 (AQP3) is overexpressed in gastric cancer tissues, whereas CDH1 is expressed in normal gastric tissues (31). It has been suggested that AQP3 induces EMT in gastric cancer cells (31). Appendiceal and intramucosal gastric signet ring cell carcinomas have been identified in diffuse-type gastric carcinoma patients with CDH1 mutations (32). Thus, whether signet ring cell carcinoma in the appendix is primary or metastatic should be carefully examined (32).
CDH1 and EMT
EMT is a switching mechanism (33). EMT typically occurs during early embryogenesis, and the mesenchymal-epithelial transition (MET), the reverse phenomenon of EMT, might also occur during the reprogramming of fibroblasts through pluripotent factors (33). Epithelial cells convert into mesenchymal cells during EMT, which involves abundant molecular network alterations (33). Smoking reportedly induces EMT in non-small cell lung cancer through the HDAC-mediated down-regulation of CDH1 (34). The mechanism of EMT in cancer should be investigated in correlation with CDH1 (34). As metastasis is one of the causes of cancer progression, metastatic stem cells, which initiate metastasis, are a noteworthy concept (35). Metastatic stem cells may be supported through a stem cell niche, such as hematopoietic stem cells, providing insight into the metastasis mechanism induced by EMT (35).
In EMT-related signal pathways in the neural crest, SMAD-interacting protein 1 (SIP1) is a key factor in CDH1 to CDH2 switching during development (36). CDH1 expression is regulated through snail family zinc finger 1 (SNAI1) (SNAIL) signaling, which induces EMT in gastric cancer (37). The amplification of ERBB2, MET, and FGFR2 is also involved in EMT induction in gastric cancer (37).
CDH1 is a major marker of epithelial cell states. In BGC823 human gastric cancer cells, CDH1 was up-regulated through the siRNA-based gene knockdown of N-acetylglucosaminyltransferase V (GnT-V) (38). When considering the expression of other EMT markers, GnT-V might contribute to the metastasis and invasion of gastric cancer (38). CDH1 is down-regulated during EMT and has been implicated in the induction of pluripotency (39,40). CDH1 is also down-regulated in human cancer and has been correlated with increased WNT expression (41).
CDH1 and cancer stem cells (CSCs)
CDH1 expression is decreased during the EMT process, which might represent an essential mechanism for CSC maintenance (42). Considering that CSCs and EMT are strongly related, the CDH1 function might also be involved in CSC development (43). A decrease in CDH1 expression in hepatocellular carcinomas has been correlated with early recurrent disease (44). CDH1 network created by cBioPortal may be useful to reveal the cancer mechanism (Figure 1, Table 1) (45,46).
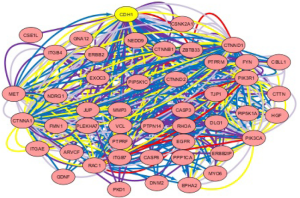
Full table
Conclusions
In conclusion, CDH1 is a key molecule for the phenotypic transition of gastric cancer cells into mesenchymal states. CDH1 is up-regulated in epithelial cells, and the down-regulation of CDH1 leads to EMT. The role of CDH1 as a marker for EMT detection and the mechanism of EMT via CDH1 and other molecular signaling should be further investigated to understand gastric cancer and CSCs.
Acknowledgements
Funding: This work was supported by grants from the National Institute of Biomedical Innovation (The Advanced Research for Medical Products Mining Program), Japan Agency for Medical Research and Development (Practical Research for Innovative Cancer Control), and the National Cancer Center Research and Development Fund. Dr. Komatsu received a research resident fellowship from the Foundation for Promotion of Cancer Research in Japan.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hamilton SR, Aaltonen LA. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press, 2000.
- Cui J, Li F, Wang G, et al. Gene-expression signatures can distinguish gastric cancer grades and stages. PLoS One 2011;6:e17819. [PubMed]
- Wang K, Yuen ST, Xu J, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet 2014;46:573-82. [PubMed]
- Tahara T, Shibata T, Okubo M, et al. DNA methylation status of epithelial-mesenchymal transition (EMT)--related genes is associated with severe clinical phenotypes in ulcerative colitis (UC). PLoS One 2014;9:e107947. [PubMed]
- Gaston D, Hansford S, Oliveira C, et al. Germline mutations in MAP3K6 are associated with familial gastric cancer. PLoS Genet 2014;10:e1004669. [PubMed]
- Torres-Martin M, Lassaletta L, Isla A, et al. Global expression profile in low grade meningiomas and schwannomas shows upregulation of PDGFD, CDH1 and SLIT2 compared to their healthy tissue. Oncol Rep 2014;32:2327-34. [PubMed]
- Nadauld LD, Garcia S, Natsoulis G, et al. Metastatic tumor evolution and organoid modeling implicate TGFBR2 as a cancer driver in diffuse gastric cancer. Genome Biol 2014;15:428. [PubMed]
- Flatley JE, Sargent A, Kitchener HC, et al. Tumour suppressor gene methylation and cervical cell folate concentration are determinants of high-risk human papillomavirus persistence: a nested case control study. BMC Cancer 2014;14:803. [PubMed]
- Chen A, Beetham H, Black MA, et al. E-cadherin loss alters cytoskeletal organization and adhesion in non-malignant breast cells but is insufficient to induce an epithelial-mesenchymal transition. BMC Cancer 2014;14:552. [PubMed]
- Cisco RM, Ford JM, Norton JA. Hereditary diffuse gastric cancer: implications of genetic testing for screening and prophylactic surgery. Cancer 2008;113:1850-6. [PubMed]
- Chen Y, Kingham K, Ford JM, et al. A prospective study of total gastrectomy for CDH1-positive hereditary diffuse gastric cancer. Ann Surg Oncol 2011;18:2594-8. [PubMed]
- Lee YS, Cho YS, Lee GK, et al. Genomic profile analysis of diffuse-type gastric cancers. Genome Biol 2014;15:R55. [PubMed]
- Black MD, Kaneshiro R, Lai JI, et al. Hereditary diffuse gastric cancer associated with E-cadherin germline mutation: a case report. Hawaii J Med Public Health 2014;73:204-7. [PubMed]
- Tanabe S, Aoyagi K, Yokozaki H, et al. Gene expression signatures for identifying diffuse-type gastric cancer associated with epithelial-mesenchymal transition. Int J Oncol 2014;44:1955-70. [PubMed]
- Guilford PJ, Hopkins JB, Grady WM, et al. E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat 1999;14:249-55. [PubMed]
- Gayther SA, Gorringe KL, Ramus SJ, et al. Identification of germ-line E-cadherin mutations in gastric cancer families of European origin. Cancer Res 1998;58:4086-9. [PubMed]
- Abedi-Ardekani B, Hainaut P. Cancers of the upper gastro-intestinal tract: a review of somatic mutation distributions. Arch Iran Med 2014;17:286-92. [PubMed]
- Chu CM, Chen CJ, Chan DC, et al. CDH1 polymorphisms and haplotypes in sporadic diffuse and intestinal gastric cancer: a case-control study based on direct sequencing analysis. World J Surg Oncol 2014;12:80. [PubMed]
- Li DM, Zhang J, Li WM, et al. MAWBP and MAWD inhibit proliferation and invasion in gastric cancer. World J Gastroenterol 2013;19:2781-92. [PubMed]
- Park JW, Jang SH, Park DM, et al. Cooperativity of E-cadherin and Smad4 loss to promote diffuse-type gastric adenocarcinoma and metastasis. Mol Cancer Res 2014;12:1088-99. [PubMed]
- Choi WS, Seo HS, Song KY, et al. Gastrokine 1 expression in the human gastric mucosa is closely associated with the degree of gastritis and DNA methylation. J Gastric Cancer 2013;13:232-41. [PubMed]
- Gao M, Li W, Wang H, et al. The distinct expression patterns of claudin-10, -14, -17 and E-cadherin between adjacent non-neoplastic tissues and gastric cancer tissues. Diagn Pathol 2013;8:205. [PubMed]
- Garziera M, Canzonieri V, Cannizzaro R, et al. Identification and characterization of CDH1 germline variants in sporadic gastric cancer patients and in individuals at risk of gastric cancer. PLoS One 2013;8:e77035. [PubMed]
- Liu XJ, Chen ZF, Li HL, et al. Interaction between cyclooxygenase-2, Snail, and E-cadherin in gastric cancer cells. World J Gastroenterol 2013;19:6265-71. [PubMed]
- Wadhwa R, Song S, Lee JS, et al. Gastric cancer-molecular and clinical dimensions. Nat Rev Clin Oncol 2013;10:643-55. [PubMed]
- Wong H, Yau T. Targeted therapy in the management of advanced gastric cancer: are we making progress in the era of personalized medicine? Oncologist 2012;17:346-58. [PubMed]
- Schneider BG, Peng DF, Camargo MC, et al. Promoter DNA hypermethylation in gastric biopsies from subjects at high and low risk for gastric cancer. Int J Cancer 2010;127:2588-97. [PubMed]
- Chan AO, Lam SK, Wong BC, et al. Promoter methylation of E-cadherin gene in gastric mucosa associated with Helicobacter pylori infection and in gastric cancer. Gut 2003;52:502-6. [PubMed]
- Leung WK, Man EP, Yu J, et al. Effects of Helicobacter pylori eradication on methylation status of E-cadherin gene in noncancerous stomach. Clin Cancer Res 2006;12:3216-21. [PubMed]
- Dong H, Guo H, Xie L, et al. The metastasis-associated gene MTA3, a component of the Mi-2/NuRD transcriptional repression complex, predicts prognosis of gastroesophageal junction adenocarcinoma. PLoS One 2013;8:e62986. [PubMed]
- Chen J, Wang T, Zhou YC, et al. Aquaporin 3 promotes epithelial-mesenchymal transition in gastric cancer. J Exp Clin Cancer Res 2014;33:38. [PubMed]
- Hamilton LE, Jones K, Church N, et al. Synchronous appendiceal and intramucosal gastric signet ring cell carcinomas in an individual with CDH1-associated hereditary diffuse gastric carcinoma: a case report of a novel association and review of the literature. BMC Gastroenterol 2013;13:114. [PubMed]
- Chen J, Han Q, Pei D. EMT and MET as paradigms for cell fate switching. J Mol Cell Biol 2012;4:66-9. [PubMed]
- Nagathihalli NS, Massion PP, Gonzalez AL, et al. Smoking induces epithelial-to-mesenchymal transition in non-small cell lung cancer through HDAC-mediated downregulation of E-cadherin. Mol Cancer Ther 2012;11:2362-72. [PubMed]
- Oskarsson T, Batlle E, Massagué J. Metastatic stem cells: sources, niches, and vital pathways. Cell Stem Cell 2014;14:306-21. [PubMed]
- Rogers CD, Saxena A, Bronner ME. Sip1 mediates an E-cadherin-to-N-cadherin switch during cranial neural crest EMT. J Cell Biol 2013;203:835-47. [PubMed]
- Katoh M. Epithelial-mesenchymal transition in gastric cancer (review). Int J Oncol 2005;27:1677-83. [PubMed]
- Huang B, Sun L, Cao J, et al. Downregulation of the GnT-V gene inhibits metastasis and invasion of BGC823 gastric cancer cells. Oncol Rep 2013;29:2392-400. [PubMed]
- Chang CC, Hsu WH, Wang CC, et al. Connective tissue growth factor activates pluripotency genes and mesenchymal-epithelial transition in head and neck cancer cells. Cancer Res 2013;73:4147-57. [PubMed]
- Redmer T, Diecke S, Grigoryan T, et al. E-cadherin is crucial for embryonic stem cell pluripotency and can replace OCT4 during somatic cell reprogramming. EMBO Rep 2011;12:720-6. [PubMed]
- Vadnais C, Shooshtarizadeh P, Rajadurai CV, et al. Autocrine Activation of the Wnt/β-Catenin Pathway by CUX1 and GLIS1 in Breast Cancers. Biol Open 2014;3:937-46. [PubMed]
- Wang Y, Shi J, Chai K, et al. The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets 2013;13:963-72. [PubMed]
- Cardiff RD, Couto S, Bolon B. Three interrelated themes in current breast cancer research: gene addiction, phenotypic plasticity, and cancer stem cells. Breast Cancer Res 2011;13:216. [PubMed]
- Xia H, Ooi LL, Hui KM. MiR-214 targets β-catenin pathway to suppress invasion, stem-like traits and recurrence of human hepatocellular carcinoma. PLoS One 2012;7:e44206. [PubMed]
- Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [PubMed]
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401-4. [PubMed]


