Hepatic angiomyolipoma, inflammatory variant: a case report and review of literature
Introduction
Hepatic angiomyolipoma (AML) is a rare benign tumor first described by Ishak in 1976 (1). Hepatic AML has been increasingly recognized of late as a result of wide-spread use of high resolution imaging techniques and many cases have been diagnosed by core needle biopsy of ambiguous liver masses (2). Owing to their significantly varied histological appearance, several histological variants of hepatic AML have been described viz. classical/mixed, leiomyomatous, lipomatous, myelolipomatous, angiomatous/angiomyomatous, epithelioid, trabecular, oncocytic, pleomorphic and inflammatory variants (2-5). Recognition of these entities is necessary to avoid misinterpretation as variants of hepatocellular carcinoma (HCC), melanoma or other primary or metastatic malignant neoplasms with prominent inflammation, particularly on core needle biopsy (2,6).
The inflammatory variant is the least common variant of hepatic AML and shows a dense lymphoplasmacytic infiltrate which sometimes obscures the true nature of the AML. Less than fifteen cases have been reported in the literature so far (7-12). Most of these cases had displayed a minor focal conventional tumor component. However, the pathogenesis of the inflammatory reaction in hepatic AML remains poorly understood.
Here we describe a new case of hepatic AML, inflammatory variant.
Case report
A 54-year-old woman presented with fatigue, vague abdominal pain and fullness, and anorexia. There was no history of fever or jaundice. On clinical examination, there was mild tenderness in the right hypochondrium and hepatomegaly (2 cm below the costal margin). No stigmata of tuberous sclerosis were seen. All the laboratory parameters were within normal limits with the exception of microcytic, hypochromic anemia (Hb: 9.8 g/dL), elevated erythrocyte sedimentation rate of 64 mm and C-reactive protein (CRP) of 12.41 mg/dL. Liver function tests and AFP levels were within normal limits. The patient was seronegative for hepatitis B, hepatitis C and HIV. Computerised tomography (CT) (plain and contrast) showed a heterogeneously enhancing, circumscribed mass lesion involving segments V and VIII of the right lobe of the liver measuring 11.5 cm × 6.4 cm × 5.7 cm, with dilated hepatic artery branches within it (Figure 1). No other abnormalities of abdominal organs were noted. Radiologically, a differential diagnosis of HCC and hemangioma was given. Ultrasound guided FNA of the lesion was inconclusive. All other clinical investigations like upper gastrointestinal endoscopy, colonoscopy and magnetic resonance cholangiopancreatography (MRCP) were within normal limits.
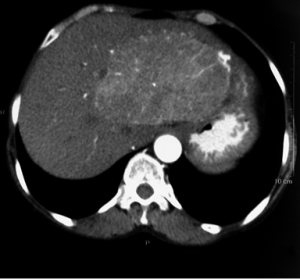
The patient underwent surgery and only a part of the neoplasm could be resected due to intraoperative complications. The resected specimen was sent for histopathological examination.
Materials and methods
Formalin fixed, paraffin embedded sections were examined with routine hematoxylin & eosin stain. IHC was performed on 3 µ sections cut from paraffin blocks according to the manufacturer’s instructions. The antibodies used are given in Table 1.
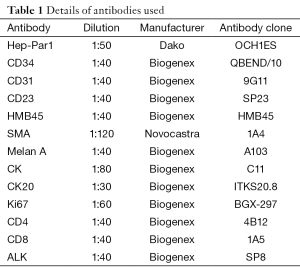
Full table
Results
Pathologic findings
Macroscopic features—the resected specimen was received in two fragments. The larger fragment measured 7 cm × 5 cm × 1.8 cm and the smaller fragment measured 2 cm × 1.5 cm × 1 cm. The external surface of both fragments was smooth. The cut surface revealed a well demarcated but unencapsulated, friable, grey brown lesion measuring 6.3 cm × 4.6 cm × 1.5 cm in the larger fragment and 1.7 cm × 1.2 cm × 0.8 cm in the smaller fragment. The cut surface of the lesion showed areas of haemorrhage and grey white areas.
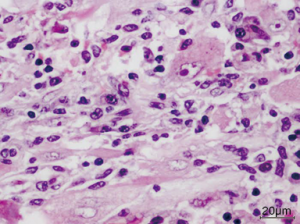
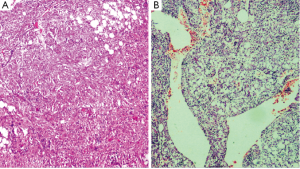
Microscopic features—the sections revealed a lesion strikingly rich in inflammatory cells composed predominantly of lymphocytes and plasma cells (Figure 2). Few groups of adipocytes interspersed amongst spindle shaped myoid cells were identified after examining multiple sections (Figure 3). Areas composed of aggregates of spindle shaped myoid cells were seen. Few Reed-Sternberg like cells were seen (Figure 4). Few entrapped, degenerating hepatocytes were also identified. There were no associated features of cirrhosis or hepatitis in the adjacent liver tissue.
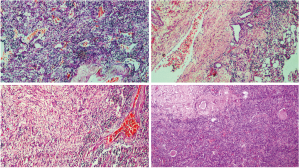
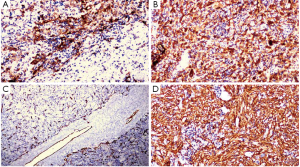
The myoid cells were positive for HMB45, Melan A (Figure 5A,B) and negative for Hep-Par1, CK7, CK20, ALK and showed a Ki67 index of 5%. A marked proliferation of blood vessels comprising of a few thick walled blood vessels and numerous irregularly distributed vascular channels which were positive for CD31 and CD34 was noted (Figure 5C). CD4 and CD8 were positive in reactive lymphoid cells. The areas of spindle cells were strongly positive for SMA (Figure 5D).
The patient did not have any major complaints after the surgery and is to be followed up on a biannual basis after the first two visits at 15 days and 2 months after the surgery. During the last visit, the patient was doing well.
Discussion
AML occurs less frequently in the liver than in the kidney. Tuberous sclerosis may be associated in 6% of cases (13-15). Various subtypes of hepatic AML have been described like classical/mixed, leiomyomatous, lipomatous, myelolipomatous, angiomatous/angiomyomatous, epithelioid, trabecular, oncocytic, pleomorphic and inflammatory variants (2-5). The inflammatory variant has prominent, dense lymphoid aggregates (16). The inflammatory variant of hepatic AML is exceedingly rare. Less than fifteen cases have been reported to date (7-12). There is a striking female predilection (F:M =7:1), with a mean age of 41 years (range, 21-63 years). Five of the lesions originated in the left lobe of the liver. None of them were associated with other liver diseases or cirrhosis, and hepatitis B and C serology was negative in two cases with available data. Unlike classical hepatic AML which may be associated with the tuberous sclerosis complex (TSC), none of the earlier reported hepatic inflammatory AML were associated with this disorder. Most were incidental findings on imaging examination and only two cases presented with constitutional symptoms (unexplained fever or general illness) (7,10). The tumor size ranged from 3 to 10 cm (mean: 6.5). None recurred or metastasized at a mean follow-up of 5.3 years (follow-up range, 2-9 years). All tumors showed a predominance of inflammatory pattern comprising more than 90% of the lesion and fat cells represented <5% of lesional cells in all cases. The presence of scattered adipocytes, sinusoidal or irregular thick-walled vessels and myoid cells were the clue to the diagnosis of AML.
In the present case of a 54-year-old woman, who presented with vague abdominal symptoms and a right lobe liver mass detected on CT scan, there was neither association with tuberous sclerosis nor hepatitis or cirrhosis. Morphologically, the lesion was strikingly rich in inflammatory cells and classical AML-like areas were identified only after examining multiple sections and were confirmed by positive IHC staining for HMB45 and Melan A.
Our case was unique as it had a very small adipocytic component due to which the diagnosis was missed on CT scan and FNAC where a primary diagnosis of HCC with a differential diagnosis of hemangioma was given. Even on histology, the first impression was of a lymphoepithelial—like HCC.
Inflammatory AML should be differentiated from other hepatic lesions with a predominance of inflammatory cells like inflammatory myofibroblastic tumor, lymphoepithelial—like HCC; and from spindle cell tumors like pleomorphic undifferentiated sarcoma. The presence of HMB45 positive myoid cells is the defining criterion of hepatic AML, which is a tumor capable of dual myomatous and lipomatous differentiation and melanogenesis (5).
In inflammatory myofibroblastic tumor (IMT), a fibrohistiocytic reaction with a predominant plasma cell component, storiform sclerosis and obliterative phlebitis are often present, as well as acutely inflamed, entrapped bile ducts (10,17). The spindled stromal cells demonstrate both IHC and structural characteristics of myofibroblasts. The cells are positive for SMA, muscle-specific actin, desmin and vimentin but negative for HMB45 and Melan A (18).
In IMT-like follicular dendritic cell tumor, in which there is a clonal proliferation of EBV-infected follicular dendritic cells (19), may resemble inflammatory AML, as histologically it is characterised by spindle cell proliferation admixed with abundant inflammatory cells, mainly lymphocytes and plasma cells (20). Follicular dendritic cells are immunoreactive with the antibodies CD21, CD35, CD23, R4/23, and KiM4 (21-23). However, the neoplastic cells in inflammatory AML are negative for CD21, CD35, CD23, R4/23 and KiM4.
Although a dense lymphocytic infiltrate is seen in lymphoepithelial—like HCC, the tumor cells are positive for Hep-Par1 and negative for melanocytic markers like Melan A and HMB45.
In pleomorphic undifferentiated sarcoma, the tumor cells are often arranged in a storiform pattern. The tumor cells are pleomorphic and bizarre with foamy cytoplasm and marked atypia. Numerous mitoses are present. The tumor cells are negative on IHC for cytokeratins, melanocytic markers, CD45, S100 and muscle markers unlike inflammatory AML which is positive for melanocytic markers HMB45 and Melan A.
It is important to identify this entity as the management of hepatic AML is conservative when the tumor is asymptomatic and less than 5 cm in size (24). Surgical resection is indicated in the following scenarios: (I) the patient is symptomatic; (II) the tumor shows an aggressive growth; (III) the tumor shows growth into the vessels evidenced by fine needle biopsy or imaging studies; (IV) the component of the tumor shows atypical epithelioid pattern, high proliferation activity, and/or p53 immunoreactivity; and (V) a definitive diagnosis cannot be made by imaging and pathological studies from malignant tumors (25).
In conclusion, inflammatory hepatic AML is a rare diagnostically challenging neoplasm but the presence of typical, focal areas of AML and expression of melanocytic markers like Melan A and HMB45, help in its delineation. Knowledge of this entity is important so that appropriate IHC is used to differentiate this tumor from morphological mimics, which would impact further management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ishak KG. Mesenchymal tumors of the liver. In: Okuda K, Peters RL, editors. Hepatocellular Carcinoma. New York: John Wiley & Sons, 1976.
- Agaimy A, Vassos N, Croner RS, et al. Hepatic angiomyolipoma: a series of six cases with emphasis on pathological-radiological correlations and unusual variants diagnosed by core needle biopsy. Int J Clin Exp Pathol 2012;5:512-21. [PubMed]
- Chang Z, Zhang JM, Ying JQ, et al. Characteristics and treatment strategy of hepatic angiomyolipoma: a series of 94 patients collected from four institutions. J Gastrointestin Liver Dis 2011;20:65-9. [PubMed]
- Nonomura A, Enomoto Y, Takeda M, et al. Angiomyolipoma of the liver: a reappraisal of morphological features and delineation of new characteristic histological features from the clinicopathological findings of 55 tumours in 47 patients. Histopathology 2012;61:863-80. [PubMed]
- Tsui WM, Colombari R, Portmann BC, et al. Hepatic angiomyolipoma: a clinicopathologic study of 30 cases and delineation of unusual morphologic variants. Am J Surg Pathol 1999;23:34-48. [PubMed]
- Zhong DR, Ji XL. Hepatic angiomyolipoma-misdiagnosis as hepatocellular carcinoma: A report of 14 cases. World J Gastroenterol 2000;6:608-12. [PubMed]
- Kojima M, Nakamura S, Ohno Y, et al. Hepatic angiomyolipoma resembling an inflammatory pseudotumor of the liver. A case report. Pathol Res Pract 2004;200:713-6. [PubMed]
- Shi H, Cao D, Wei L, et al. Inflammatory angiomyolipomas of the liver: a clinicopathologic and immunohistochemical analysis of 5 cases. Ann Diagn Pathol 2010;14:240-6. [PubMed]
- Liu Y, Wang J, Lin XY, et al. Inflammatory angiomyolipoma of the liver: a rare hepatic tumor. Diagn Pathol 2012;7:122. [PubMed]
- Agaimy A, Märkl B. Inflammatory angiomyolipoma of the liver: an unusual case suggesting relationship to IgG4-related pseudotumor. Int J Clin Exp Pathol 2013;6:771-9. [PubMed]
- Sun K, Zhao M, Yao H, et al. Premelanosome-negative inflammatory angiomyolipoma of liver with expression of cathepsin K and TFE3. Int J Clin Exp Pathol 2014;7:8170-5. [PubMed]
- C R J, Menon DP, Augustine J, et al. Epithelioid Angiomyolipoma of Liver with an Inflammatory Component: A Case Report. Case Reports Hepatol 2013;2013:738708.
- Ben-Izhak O, Groissman G, Lichtig C. Hepatic angiomyolipoma in childhood: association with tuberous sclerosis. Pediatr Pathol Lab Med 1995;15:213-7. [PubMed]
- Robinson JD, Grant EG, Haller JO, et al. Hepatic angiomyolipomas in tuberous sclerosis. Report of two cases. J Ultrasound Med 1989;8:575-8. [PubMed]
- Nonomura A, Mizukami Y, Kadoya M. Angiomyolipoma of the liver: a collective review. J Gastroenterol 1994;29:95-105. [PubMed]
- Petrolla AA, Xin W. Hepatic angiomyolipoma. Arch Pathol Lab Med 2008;132:1679-82. [PubMed]
- Yamaguchi J, Sakamoto Y, Sano T, et al. Spontaneous regression of inflammatory pseudotumor of the liver: report of three cases. Surg Today 2007;37:525-9. [PubMed]
- Coffin CM, Watterson J, Priest JR, et al. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 1995;19:859-72. [PubMed]
- Cheuk W, Chan JK, Shek TW, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol 2001;25:721-31. [PubMed]
- Kim HJ, Kim JE, Kang GH, et al. Inflammatory Pseudotumor-like Follicular Dendritic Cell Tumor of the Spleen with Extensive Histiocytic Granulomas and Necrosis: A Case Report and Literature Review. Korean J Pathol 2013;47:599-602. [PubMed]
- Perez-Ordoñez B, Rosai J. Follicular dendritic cell tumor: review of the entity. Semin Diagn Pathol 1998;15:144-54. [PubMed]
- Shek TW, Ho FC, Ng IO, et al. Follicular dendritic cell tumor of the liver. Evidence for an Epstein-Barr virus-related clonal proliferation of follicular dendritic cells. Am J Surg Pathol 1996;20:313-24. [PubMed]
- Chan JK. Proliferative Lesions of Follicular Dendritic (Cells: An Overview, Including a Detailed Account of Follicular Dendritic Cell Sarcoma, A Neoplasm with Many Faces and Uncommon Etiologic Associations. Adv Anat Pathol 1997;4:387-411.
- Yang CY, Ho MC, Jeng YM, et al. Management of hepatic angiomyolipoma. J Gastrointest Surg 2007;11:452-7. [PubMed]
- Kamimura K, Nomoto M, Aoyagi Y. Hepatic angiomyolipoma: diagnostic findings and management. Int J Hepatol 2012;2012:410781.


