Gastric carcinoid type III—appearances can be deceptive!
Introduction
Neuroendocrine tumors (NETs) arise from neuroendocrine cells found throughout the body. In United States, incidence of these tumors rose from 1.09 to 5.25 cases per 100,000 between 1973 and 2004 (1). NETs of the stomach (gastric carcinoids) are rare tumors which comprise less than 1% of all gastric neoplasms (2). Most of these tumors arise from enterochromaffin-like (ECL) cells of the fundic gastric glands and are classified into three subtypes. Type I and II gastric carcinoid tumors have benign course and rarely metastasize while type III carcinoid tumors have a more aggressive course and generally metastasize before primary is diagnosed (3). Type III gastric carcinoids can pose diagnostic, prognostic and therapeutic challenges when not associated with significant clinical symptoms. Even histopathology of such lesions can sometimes not provide any prognostic clue especially when tumor is confined to mucosa and have a bland morphology. We herein present a case of gastric carcinoid type III in a 68-year-old male in which we encountered similar challenges.
Case report
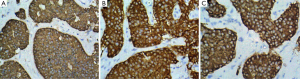
A 68-year-old male patient presented with a single episode of massive upper GI bleed. His vitals were stable. Hemogram and routine biochemistry were within normal limits. USG-abdomen showed few enlarged gastro-hepatic and peripancreatic lymph-nodes. CECT-abdomen showed multiple small mildly enhancing lesions in liver and spleen with few enlarged lymph nodes, likely metastatic. PET scan revealed low FDG avid disease involving liver, spleen, lung, bone and abdominal lymph nodes, likely metastatic. Upper GI Endoscopy was performed which revealed a single large gastric ulcer in fundus of stomach. Endoscopic biopsy from the stomach lesion showed small nodular aggregates (>5 cells) of monomorphic cells measuring around 2 mm in largest diameter, with scant mitotic figures (Figure 1A). In spite of the subtle findings, a diagnosis of intramucosal carcinoid was considered over enterochromaffin cell hyperplasia, because of the number of cells and size of the aggregates. Subsequently, biopsy from liver nodule was performed which showed small, nodular aggregates and trabeculae of similar cells infiltrating in liver parenchyma (Figure 1B,C). The cells here showed a clear neuroendocrine morphology with Ki-67 score of 7-8%. Immunohistochemistry was performed on both lesions which showed strong positivity for chromogranin, synaptophysin, NSE and CD56 (Figure 2). Serum Biomarker study revealed serum gastrin 75 ng/mL, chromogranin 790.97 ng/mL and NSE 43.48 ng/mL. A final diagnosis of gastric carcinoid type III with liver metastasis was made after reviewing the literature. The patient received six cycles of chemotherapy which he tolerated well and was discharged in stable condition after 6 months.

Discussion
Gastrointestinal carcinoid tumors are rare tumors that originate from ECL cells in the GI mucosa. The stomach is the least common site of gastrointestinal carcinoids. Around 45% of GI carcinoids arise in the small intestine, 20% in the rectum, 16% in the appendix, 12% in the colon, and only 7% in the stomach (4). The incidence of gastric carcinoids has been shown to be increasing. Whether this represents a true biological increase in the disease or reflects a change in awareness and increase in reporting of these tumors remains unclear. Three types of gastric carcinoids have been described depending on the tumor characteristics, histology, association with hypergastrinemia, and their biological behavior. Type I is associated with chronic atrophic gastritis, type II develops in patients with combined multiple endocrine neoplasia type I and the Zollinger–Ellison syndrome. Type III carcinoids account for around 20% of the cases and are known as sporadic gastric carcinoids as they show no association with hypergastrinemia, chronic atrophic gastritis, or Zollinger-Ellison syndrome. They occur most frequently in males over the age of 50 years in contrast to other subtypes which are more common in females. They usually present as large solitary lesions (>2 cm in diameter) and are often metastatic upon diagnosis. Most commonly, these lesions are found incidentally on endoscopy. Some patients present with nonspecific symptoms such as nausea, vomiting, dyspepsia, abdominal discomfort, or with complications such as gastrointestinal bleeding (5). The index case presented with massive GI bleeding. Histologically, these tumors are thought to be derived from enterochromaffin like cells, or X cells. In contrast to type I and type II carcinoids, these tumors evolve without evidence of hyperplasia or dysplasia in adjacent mucosa, and generally invade underlying submucosa/deeper tissue in more than 75% of cases (6). However, in cases where lesion is largely limited to mucosa, with bland morphology on histopathology, low mitotic count and low ki-67 score, these cases can be difficult to differentiate from type I and type II carcinoids and one may falsely categorize these lesions in more indolent types. Also, in cases where tumor is arranged largely in small aggregates of cells, it may be difficult to differentiate it from endocrine cell hyperplasia where aggregates are smaller than 0.5 mm and comprising less than 5 cells each.
Plasma chromogranin A appears to be a valuable tumor marker for all types of gastric carcinoids. Chromogranin A levels are reported to be increased in almost all patients with type III carcinoids and the levels are higher than those with type I/II lesions. It has also been reported that plasma chromogranin A concentration correlates with tumor size, is an independent predictor of survival and can be useful in following response to therapy (7). Type III gastric carcinoids should be treated similar to adenocarcinoma of the stomach, with an enbloc resection and an appropriate lymph node clearance. Regression of type III carcinoid has been reported after octreotide treatment in patients with normal gastrin levels and octreotide has also been shown to reduce the size of metastases (8). Biologically these are aggressive tumors and show regional lymph node involvement in up to 55% of cases and liver metastases in over 2/3 of the cases. The 5-year survival is <35% but, in patients with distant metastases, it is only 10% (9).
Conclusions
Subtyping gastric carcinoids is helpful in the prediction of malignant potential and long-term survival and is a guide to management. Patients with type III gastric carcinoids usually present with metastatic disease and have significantly shorter survival than type I and type II patients. Histopathological assessment should be supplemented by Immunohistochemical and Serum Biomarker studies along with clinical correlation to reach at a definitive diagnosis. High index of suspicion is needed even in cases presenting with lesion limited to mucosa and having a bland morphology.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [PubMed]
- Delle Fave G, Capurso G, Milione M, et al. Endocrine tumours of the stomach. Best Pract Res Clin Gastroenterol 2005;19:659-73. [PubMed]
- Rindi G, Bordi C, Rappel S, et al. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg 1996;20:168-72. [PubMed]
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59. [PubMed]
- Wardlaw R, Smith JW. Gastric carcinoid tumors. Ochsner J 2008;8:191-6. [PubMed]
- Klöppel G, Anlauf M. Epidemiology, tumour biology and histopathological classification of neuroendocrine tumours of the gastrointestinal tract. Best Pract Res Clin Gastroenterol 2005;19:507-17. [PubMed]
- Granberg D, Wilander E, Stridsberg M, et al. Clinical symptoms, hormone profiles, treatment, and prognosis in patients with gastric carcinoids. Gut 1998;43:223-8. [PubMed]
- Tomassetti P, Migliori M, Caletti GC, et al. Treatment of type II gastric carcinoid tumors with somatostatin analogues. N Engl J Med 2000;343:551-4. [PubMed]
- Modlin IM, Kidd M, Latich I, et al. Current status of gastrointestinal carcinoids. Gastroenterology 2005;128:1717-51. [PubMed]


