Progress with each passing day: role of endoscopy in early gastric cancer
Gastric cancer continues to be an important healthcare problem, altogether 989,000 new gastric cancer cases are estimated to arise annually worldwide, and 42% of them are in China (1,2). In Europe and the United States, 5-year survival rate of gastric cancer remains poor, which does not exceed 25% (3). Advanced gastric cancer, especially stage IV patients have a lower 5-year survival rate than 4% (4), whereas early gastric cancer can reach a 95% of 5-year survival rate (5). Treatments for gastric cancer patients with more advanced stage are unsatisfactory. The problem of late diagnosis is due to a substantial proportion of patients with early stage disease being asymptomatic. Early diagnoses and interventions are the keys to decrease mortality and improve survival, in that case in the management of early gastric cancer (EGC); a major role is played by endoscopy.
EGC is defined as gastric cancer in which tumor invasion is confined to the mucosa or submucosa (T1 cancer), irrespective of lymph node status (6). Advances in diagnostic and treatment technology have contributed to a trend towards minimal invasive surgery such as endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD).
In order to heighten the standards of diagnosis and treatment of EGC, tremendous endoscopic techniques, materials, and devices had emerged. This review addresses the endoscopic diagnosis and treatment for EGC.
Endoscopic diagnoses in EGC
The endoscopic diagnosis of EGC needs two steps, detection and characterization. Detection is achieved by conventional white light endoscopy and characterization by magnifying endoscopy, magnifying endoscopy with narrow-band imaging (M-NBI) and other advanced methods.
Conventional white light endoscopy
During the endoscopy, in order to avoid blind spots we highly recommend to follow a systematic screening protocol for the stomach (SSS), which was proposed by Yao (7). It is necessary to pay attention to the change of gastric mucosa, especially the ones differ from the background mucosa, such as polyp, ulceration, reddish, erosion, and discontinue of the mucosal wrinkles. According to the Paris classification of superficial neoplastic lesions, EGC can be divided into three categories: protruding (0-I), nonprotruding and nonexcavated (0-II), and excavated (0-III). Type 0-II lesions are then subdivided into slightly elevated (IIa), flat (IIb), or depressed (IIc) (8). This classification is widely used for describing the lesions, but it isn’t able to conform the character and invasion of the lesion unfortunately. To gain a better understanding of the lesions has brought the emergence of more advanced endoscopic techniques.
Magnifying endoscopy
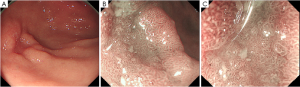
The magnifying endoscopy can amplify the lesion to about 100 times, with a high articulation and resolution instead of largen the picture only. Pathologic slides observe the tissue structure of longitudinal section and the cytological morphology, yet magnifying endoscopy can vertically observe the surface structure of the mucosa and microvascular patterns. Tanaka et al. (9) described the features of EGC and classified the surface patterns of gastric tumor and the surrounding mucosa into five types: type I, small round pits of uniform size and shape; type II, slit-like pits; type III, gyrus and villous patterns; type IV, irregular arrangements and sizes of pattern types I, II, and III; type V, destructive patterns of type I, II and III. Differentiated tubular adenocarcinomas mostly are type IV surface patterns, and all of the signet-ring cell carcinomas and poorly differentiated tubular adenocarcinomas showed type V.
Magnifying endoscopy with narrow-band imaging (M-NBI)
Yao (7) demonstrated the basic principles of diagnosing EGC by endoscopy (Figure 1). After detecting the suspicious lesion through conventional white light endoscopy, we need to characterize the lesion. Fujiwara et al. (10) indicated that M-NBI has greater sensitivity and reproducibility than chromoendoscopy (CE) for the diagnosis of minute gastric cancers. With M-NBI we can characterize small or flat EGC, by clearly visualizing both the microvascular pattern and microsurface pattern (11). Yao et al. (12) also demonstrated the VS classification system. The main idea is summarized as regular, irregular, or absent microvascular/microsurface pattern. The technology of magnifying endoscopy and narrow-band imaging have brought a great help in characterizing and diagnosing the lesion.
Endoscopic treatment of EGC
Considering the adverse consequences of lymph node metastasis, gastrectomy with lymph node dissection had been the gold standard treatment, including EGC. Recently, endoscopic resection for EGC is widely accepted as one of the standard treatments together with surgical treatment.
Endoscopic resection is comparable in many respects to conventional surgery, especially its advantages of being less invasive and more economical. The extremely low incidence of lymph involvement in certain stages of EGC means such local treatment can be accomplished in selected cases. Endoscopic resection allows complete pathological staging of the cancer, which is critical, as this allows stratification and refinement of further treatment (13). Relative to the preoperative risks associated with surgery, patients who have a lower risk of developing lymph node metastasis are the ideal candidates for endoscopic resection (14).
In 2000, Gotoda et al. (15) analyzed the pathology of EGC traditional surgery, among 1,230 well differentiated intra-mucosal carcinoma lesions which were smaller than 3 cm regardless of ulceration; there were no lymph node metastasis. None of the above with less than 30-mm-diameter regardless of ulceration findings were associated with metastases. And none of the other 929 lesions without ulceration were associated with nodal metastases regardless of tumor size. He also analyzed 145 differentiated adenocarcinomas of less than 30-mm-diameter without lymphatic or venous permeation, provided that the lesion had invaded less than 500 µm into the submucosa, none of them were associated with lymph node metastasis. Kunisaki et al. (16) retrospectively evaluated 573 patients with histologically poorly differentiated type EGC (269 mucosal and 304 submucosal), and lymph node metastasis was observed in 74 patients (12.9%). Hirasawa et al. (17) investigated 3,843 patients (2,163 intramucosal cancers and 1,680 submucosal invasive cancers) who had undergone gastrectomy with lymph node dissection for solitary undifferentiated type EGC. Only 105 (4.9%) intramucosal cancers compared with 399 (23.8%) submucosal invasive cancers were associated with lymph node metastases. Both of these studies implied that a histologically poorly differentiated type mucosal gastric cancer measuring less than 20 mm without lymph vascular invasion has a lower rate of lymph node metastasis. Hanaoka et al. (18) histologically classified 376 cases of gastric cancer patients, indicated that a depth of invasion of no more than 500 µm or more from the lower margin of the muscularis mucosae (SM1), no lymphatic invasion, a tumor size of no more than 30 mm, and a proportion of undifferentiated components below 50% has a low risk of metastasis.
Patients who have a lower risk of metastasis are the ideal candidates for endoscopic treatment, by which can hopefully achieve a cure of EGC. Recent advances, including the categorizing of endoscopic resection as standard EMR or ESD will be described as below.
Endoscopic mucosal resection (EMR)
Learning from the successful application of polypectomy used to remove early colon cancer, EMR technique called the “strip biopsy” for EGC was first described in 1984, which is to lift the lesion with a grasper and to remove the lesion using a double-channel endoscope after submucosal injection of saline/diluted epinephrine solution under the lesion. In this technique, after the injection of hypertonic saline and diluted epinephrine, the periphery of the lesion is cut by a needle knife. The lesion is then removed by a snare. EMR allowed increased precision to be applied, thus permitting the entire lesion to be removed en bloc. However, the technique also requires considerable skills, and the use of the needle knife increases risk for perforation.
In 1993, a new method of EMR with a cap-fitted panendoscope (EMR-C) was developed and applied on EGC (19). The technique uses a clear plastic cap connected to the standard endoscope tip. A specialized crescent-shaped snare is deployed in the groove at the tip of the cap after the submucosal injection. Then the lesion is sucked into the cap while the snare is closed. Thus, resection can be safely performed through the submucosal layer under the lesion (20). Another technique is EMR with ligation (EMR-L), which utilizes a standard endoscopic variceal ligation device to capture the lesion, and then deploy the band underneath it to make it into a polypoid lesion (21). EMR-C and EMR-L have the advantage of being comparatively simple, using of the standard endoscope and no necessary for an additional assistant. These techniques helped to resect the lesion more efficiently and safely. It also lowered the risk of main complications, such as perforation and bleeding.
Absolute indication of EMR for EGC was published by Japanese Gastric Cancer Association in 1998: (I) well-differentiated elevated cancers less than 2 cm in diameter and (II) small (<1 cm) depressed lesions without ulceration. Also, these lesions must be moderately or well-differentiated cancers confined to the mucosa and have no lymphatic or vascular involvement (6,22). Choi et al. (23) compared the long-term outcomes after EMR and surgery. The 5-year overall survival rates and recurrence rates did not differ significantly between the EMR and surgery groups (93.6% vs. 94.2% and 1.2% vs. 1.1%).
A major limitation of EMR is incomplete resection of the lesions larger than 15 mm in one piece due to the size limitations of accessories such as snares, caps and ligating devices (24,25). Piecemeal resection also leads to a high risk of local recurrence (up to 36.5%) (26-29). For overcoming the size limitations for en bloc resection of EGC, an improvement in techniques was developed.
Endoscopic submucosal dissection (ESD)
Because of its advantage as minimal invasion, safety, and efficiency, ESD is widespread, as a way of EGC surgeries (30). Our endoscopy center, Zhongshan Hospital of Fudan University, Shanghai, China started ESD from 2006. ESD has an expanded indication as it is able to remove bigger lesions with an en bloc. The expanded ESD criteria for EGC are differentiated type cancers without evidence of lymphovascular invasion, including: (I) mucosal cancer without ulceration, irrespective of tumor size; (II) mucosal cancer with ulceration, less than 3 cm in diameter; and (III) minimal (500 µm from the muscularis mucosa) submucosal invasive cancer less than 3 cm in size. After ESD, the patients with EGC of expanded indication may be followed closely without surgery because they have very small risks for lymph node metastasis (15,31-33).
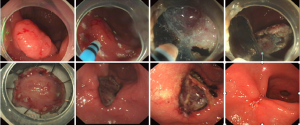
Ways of procedure may vary slightly among different institutions. Zhongshan Hospital routine is described as below (Figure 2):
- Observe the lesion’s size, location, color, and surface conditions, with or without ulceration and evaluate the depth of invasion;
- Marking dots were made approximately 5-10 mm from the lesion by argon plasma coagulation (APC);
- Several milliliters of solution (100 mL saline, 5 mL 0.8% indigo carmine, and 1 mL of epinephrine) were injected with a 23-gauge disposable needle around the lesion;
- Utilize an IT knife or a hook knife to cut the mucosa initially along the marked points;
- Submucosal connective tissue beneath the lesion was gradually dissected with the help of the transparent cap. The solution was injected repeatedly during the dissection whenever necessary. Direct dissection of the submucosal layer was carried out until complete removal had been achieved;
- Exposed vessels on the artificial ulcer were coagulated with APC or hot biopsy forceps to prevent delayed bleeding, and metallic clips were always used to close the deeply dissected areas;
- Tissue specimens were fixed to a plastic foam plate using thin needles along their edges and were then fixed in formalin solution. Procession of the resected specimens and histopathological evaluations were performed after endoscopic resection by highly experienced pathologists;
- Patients were allowed oral intake after passing gas. Antibiotics and hemocoagulase injections were applied after the procedure routinely.
We declare the prevention of infection, as the procedure time is long, post operative wound is relatively big, there will be a great potential of infection after ESD surgeries. Patients are requested to follow up with endoscopy at 1, 2, 6, and 12 months after the last endoscopic resection, and annually thereafter.
With the accumulation of clinical data, both Japan and Korea had reported long-term outcomes of ESD for EGC. Chung et al. (34) analyzed 1,000 EGC lesions treated with ESD from January 2006 to June 2007. The rates of en bloc resection, complete en bloc resection, vertical incomplete resection, and piecemeal resection were 95.3%, 87.7%, 1.8% and 4.1%, respectively. The rates of delayed bleeding, significant bleeding, perforation, and surgery related to complication were 15.6%, 0.6%, 1.2% and 0.2%, respectively. The rates of en bloc resection differed significantly in relation to the location of the lesions, presence of a scar, and histologic type. Isomoto et al. (35) studied 589 EGC patients treated with ESD from January 2001 to December 2007, en bloc resection was achieved in 94.9% and 550 of 581 lesions (94.7%) were deemed to have undergone curative resection. The 5-year overall and disease-specific survival rates were 97.1% and 100%, respectively. En bloc resection was the only significant contributor to curative ESD.
The key to improving therapeutic outcomes for EGC is early detection and accurate diagnosis. Several advances in diagnostic endoscopy including magnifying endoscopy, and narrow-band imaging have improved in tissue characterization by detailed imaging of the microvascular pattern and microsurface pattern. According to previous studying and our years of experience, ESD is an effective and safe therapy in the management of EGC. The early results so far have been encouraging, although the long-term outcome data are still being monitored. We now need to continue progress in this field to provide more outcomes and simplified techniques.
Acknowledgements
Funding: This work was supported by grants from the Youth Foundation of National Natural Science Foundation of China (81502000), the Shanghai Municipal Science and Technology Committee grant (15ZR140680013DZ1940402, 13411950800, 13411951600), and the Open Fund of Key Laboratory of Carcinogenesis and Cancer Invasion, Fudan University, Ministry of Education (KLCCI2014-6).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [PubMed]
- Gondos A, Bray F, Brewster DH, et al. Recent trends in cancer survival across Europe between 2000 and 2004: a model-based period analysis from 12 cancer registries. Eur J Cancer 2008;44:1463-75. [PubMed]
- Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 2010;17:3077-9.
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006;12:354-62. [PubMed]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer 1998;1:10-24. [PubMed]
- Yao K. The endoscopic diagnosis of early gastric cancer. Ann Gastroenterol 2013;26:11-22. [PubMed]
- Endoscopic Classification Review Group. Update on the paris classification of superficial neoplastic lesions in the digestive tract. Endoscopy 2005;37:570-8. [PubMed]
- Tanaka K, Toyoda H, Kadowaki S, et al. Features of early gastric cancer and gastric adenoma by enhanced-magnification endoscopy. J Gastroenterol 2006;41:332-8. [PubMed]
- Fujiwara S, Yao K, Nagahama T, et al. Can we accurately diagnose minute gastric cancers (≤5 mm)? Chromoendoscopy (CE) vs magnifying endoscopy with narrow band imaging (M-NBI). Gastric Cancer 2015;18:590-6. [PubMed]
- Yao K, Takaki Y, Matsui T, et al. Clinical application of magnification endoscopy and narrow-band imaging in the upper gastrointestinal tract: new imaging techniques for detecting and characterizing gastrointestinal neoplasia. Gastrointest Endosc Clin N Am 2008;18:415-33. vii-viii. [PubMed]
- Yao K, Iwashita A, Kikuchi Y, et al. Novel zoom endoscopy technique for visualizing the microvascular architecture in gastric mucosa. Clin Gastroenterol Hepatol 2005;3:S23-6. [PubMed]
- Hull MJ, Mino-Kenudson M, Nishioka NS, et al. Endoscopic mucosal resection: an improved diagnostic procedure for early gastroesophageal epithelial neoplasms. Am J Surg Pathol 2006;30:114-8. [PubMed]
- Ludwig K, Klautke G, Bernhard J, et al. Minimally invasive and local treatment for mucosal early gastric cancer. Surg Endosc 2005;19:1362-6. [PubMed]
- Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-225. [PubMed]
- Kunisaki C, Takahashi M, Nagahori Y, et al. Risk factors for lymph node metastasis in histologically poorly differentiated type early gastric cancer. Endoscopy 2009;41:498-503. [PubMed]
- Hirasawa T, Gotoda T, Miyata S, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 2009;12:148-52. [PubMed]
- Hanaoka N, Tanabe S, Mikami T, et al. Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy 2009;41:427-32. [PubMed]
- Inoue H, Takeshita K, Hori H, et al. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc 1993;39:58-62. [PubMed]
- Matsuzaki K, Nagao S, Kawaguchi A, et al. Newly designed soft prelooped cap for endoscopic mucosal resection of gastric lesions. Gastrointest Endosc 2003;57:242-6. [PubMed]
- Akiyama M, Ota M, Nakajima H, et al. Endoscopic mucosal resection of gastric neoplasms using a ligating device. Gastrointest Endosc 1997;45:182-6. [PubMed]
- Tsujitani S, Oka S, Saito H, et al. Less invasive surgery for early gastric cancer based on the low probability of lymph node metastasis. Surgery 1999;125:148-54. [PubMed]
- Choi KS, Jung HY, Choi KD, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc 2011;73:942-8. [PubMed]
- Korenaga D, Haraguchi M, Tsujitani S, et al. Clinicopathological features of mucosal carcinoma of the stomach with lymph node metastasis in eleven patients. Br J Surg 1986;73:431-3. [PubMed]
- Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's esophagus. Gastroenterology 2000;118:670-7. [PubMed]
- Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer 2007;10:1-11. [PubMed]
- Nakamoto S, Sakai Y, Kasanuki J, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy 2009;41:746-50. [PubMed]
- Horiki N, Omata F, Uemura M, et al. Risk for local recurrence of early gastric cancer treated with piecemeal endoscopic mucosal resection during a 10-year follow-up period. Surg Endosc 2012;26:72-8. [PubMed]
- Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc 2006;64:877-83. [PubMed]
- Hotta K, Oyama T, Akamatsu T, et al. A comparison of outcomes of endoscopic submucosal dissection (ESD) For early gastric neoplasms between high-volume and low-volume centers: multi-center retrospective questionnaire study conducted by the Nagano ESD Study Group. Intern Med 2010;49:253-9. [PubMed]
- Ono H, Yao K, Fujishiro M, et al. Guidelines for ESD and EMR for Early Gastric Cancer. Dig Endosc 2015. [Epub ahead of print].
- Ahn JY, Jung HY, Choi KD, et al. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc 2011;74:485-93. [PubMed]
- Kang HJ, Kim DH, Jeon TY, et al. Lymph node metastasis from intestinal-type early gastric cancer: experience in a single institution and reassessment of the extended criteria for endoscopic submucosal dissection. Gastrointest Endosc 2010;72:508-15. [PubMed]
- Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc 2009;69:1228-35. [PubMed]
- Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut 2009;58:331-6. [PubMed]