Prophylactic surgery for gastrointestinal malignancies
Introduction
Genetic syndromes are thought to be responsible for 5-10% of cancers worldwide (1,2). A number of these lead to gastrointestinal malignancies. Currently, no genetic therapy or alteration of the genome exists to treat these conditions. In the absence of systemic disease, solid tumors of the gastrointestinal tract are most effectively treated by surgical resection (1). As a natural extension of the treatment of early stage disease, increasingly surgeons are being asked to council patients harboring a deleterious mutation linked to GI malignancy on the risks and benefits of prophylactic surgery prior to any measurable disease. The objective of prophylactic surgery is to prevent or greatly reduce the risk of malignancy developing in at risk individuals. The risk reduction is balanced against the potential morbidity and mortality of the prophylactic procedure. The most common indications for prophylactic gastrointestinal surgery are adenomatous polyposis coli (APC) gene mutations for familial adenomatous polyposis (FAP) syndrome, attenuated familial adenomatous polyposis (AFAP) syndromes, and CDH1 mutations for hereditary diffuse gastric cancer (HDGC). Hereditary nonpolyposis colorectal cancer (HNPCC) or Lynch syndrome (LS) may also have indications for prophylactic surgery. The improvement of surgical technique and the increasing use of minimally invasive techniques have decreased complications and hospital stay and likely increased patient acceptance of these procedures. This review intends to identify the hereditary syndromes that can benefit from consideration of prophylactic GI surgery. The various surgical options for each disease state will be discussed including such details, as the timing of surgery, morbidity/mortality, effectiveness, postoperative surveillance needs and quality of life (QOL) following surgery.
Hereditary diffuse gastric cancer (HDGC)
HDGC is an autosomal dominant inherited syndrome primarily associated with the development of diffuse gastric cancer (DGC). The identified mutation affects the gene encoding for the E-cadherin protein (CDH1). Mutation carriers have a greater than 70% risk of developing (DGC) (3). Prophylactic total gastrectomy (PTG) represents the only viable intervention to prevent development of DGC in these patients. E-cadherin also plays an important role in the development of lobular breast cancer (LBC) and females with a CDH1 mutation are also at increased risk.
Epidemiology
Gastric cancer is divided histologically between intestinal-type, related to H. pylori and environmental factors, and diffuse-type, which is related to host factors, and genetics (4,5). Approximately 10% of gastric cancers are familial in origin. Li-Fraumeni syndrome, FAP, Peutz-Jeghers syndrome (PJS), LS and a mutation in the CDH1 gene have all been associated with an increased risk of gastric cancer (6). However, the overall lifetime risk of gastric cancer is still low in most of these genetic syndromes (3). Mutations in the CDH1 gene, which is located on chromosome 16q22.1, make up 1-3% of familial associated gastric cancers and the syndrome is called HDGC (7,8). This genetic mutation has high penetrance with a mean age of developing gastric cancer at age 38 and an overall lifetime risk of 67% in males and 83% in females (9,10). Of the patients who meet diagnostic criteria for HDGC, 30% possess CDH1 gene mutation. More than 100 different mutations of the CDH1 gene have been identified worldwide (11,12). There is worldwide distribution of HDGC, with the highest incidence present in Japan (13).
Pathophysiology
Mutation of the tumor suppressor CDH1 gene results in the loss of function of the protein E-cadherin, which is responsible for cell-cell adhesion (14). This loss of function impairs cell proliferation pathways ultimately leading to adenocarcinoma. The mutation is inherited via autosomal dominant distribution (7). LBC also has an association with loss of E-cadherin, with lifetime risks reported between 40-60% for women (10,15).
Diagnosis
The International Gastric Cancer Linkage Consortium (IGCLC) has developed diagnostic criteria for HDGC, which includes at least two cases of DGC in first or second-degree relatives, one of which occurs prior to age 50, or three documented cases regardless of age (10,16,17). The original criteria was later broadened to include DGC in individuals less than 40 years of age without a family history, and individuals and families with diagnoses of both DGC and LBC in which one of the cases is less than 50 years of age (10). Other groups have also established guidelines with slight variations from above, for who should be considered for genetic testing for CDH1 (6). Identification of high-risk families via gene identification should prompt testing in relatives and consideration of intervention. The management of patients with a history suggestive of HDGC but without a germline CDH1 mutation has not been established. The IGCLC recommends that intensive endoscopic surveillance should also be offered to these patients and their families (10).
Treatment
Preoperative endoscopic surveillance has not been effective in detecting early cancer. A series of 23 patients with HDGC underwent endoscopic surveillance with failure to detect cancer in 91% of patients (18). Specifically, in a study examining patients undergoing prophylactic gastrectomy, preoperative chromoendoscopy was unable to identify cancers 4 mm or less (19). Various institutions have reported normal preoperative endoscopic examinations, while adenocarcinoma was later confirmed after pathologic sectioning of the gastrectomy specimen (4,20). Fujita et al. demonstrate that endoscopic biopsy remains a challenging diagnostic gold standard to maintain, and should not be offered in place of surgical therapy (21). Because of the failure of endoscopic surveillance, prophylactic surgery has become the recommended intervention.
For patients who meet criteria for HDGC and test positive for mutation of the CDH1 gene, PTG is recommended beginning at age 20 due to low risk of metastasis before this age (6,9,10,22). Another group recommends that PTG occur 5 years earlier than the age of onset of the youngest relative, but not before 16 years of age for nutritional and psychosocial reasons (23). The general consensus is that PTG is probably inappropriate until age 20 and surveillance can be undertaken beginning 5 years prior age of youngest case until the patient reaches age 20 (6). A prospective study from Stanford of 18 patients with CDH1 mutations revealed unrecognized cancer in 12/13 asymptomatic subjects after pathologic sectioning. However, all of these cancers were stage I and there have been no recurrences in this group with 37 months of follow-up. In contrast, the five symptomatic patients had higher stages and only one remained free of disease at the time of follow-up. Based on these results the authors recommend early prophylactic treatment (8). Total gastrectomy with D1 node dissection is the recommended procedure and can be performed entirely laparoscopically (8,24). Given the lack of nodal metastases seen in asymptomatic patients extensive lymphadenectomy seems unwarranted (8,20). The method of reconstruction after gastrectomy is left to the discretion of the operating surgeon, but complex reconstructions can result in increased complication rates (24). Less than a total gastrectomy is inappropriate and it is essential to document the complete removal of gastric mucosa by pathologic conformation of rims of esophageal and duodenal mucosa at the two ends of the specimen (3). HDGC does not have a predisposition for specific areas; although reports of a dominant focus of cancer in the proximal third of the stomach have been reported (25). Additionally, metachronous cancers can develop in the remaining stomach (6,16,26).
Surveillance
Previously we noted that endoscopic surveillance in known carriers of a mutation is ineffective at identifying early cancers. However surveillance has a role in very young patients until they reach maturation, carriers declining PTG, and in patients meeting criteria for HDG but not carrying a known deleterious mutation. The recommendation is annual endoscopy with using high-resolution white-light endoscopy. All endoscopically visible lesions are targeted and in addition, at least six random biopsies be taken from the antrum, transitional zone, body, fundus and cardia. This results in a minimum of 30 biopsies (10). Autofluorescence and narrow-band imaging (NBI) are probably of limited additional value (21). There is no endoscopic surveillance needed after PTG.
Female carriers of the CDH1 mutation have an increased risk of LBC. The lifetime risk is approximately 40-60%. Various groups have slightly different recommendations for breast screening in CDH1 mutation carriers. IGCLC recommends monthly breast self-examination, in addition to annual mammogram and breast MRI, beginning at 35 years of age. The Stanford cancer center recommends annual screening but beginning at age 25 (10,23). Currently, there is no indication for prophylactic mastectomy in asymptomatic carriers of a CDH1 mutation (3). Future studies to better quantify the risk may identify patients in whom prophylactic mastectomy may be appropriate. There are reported associations with CDH1 mutations and colorectal cancer (CRC), but not enough data exists to support additional colorectal screening (20).
Complications and QOL
Complications associated with elective total gastrectomy include bleeding, infection, stricture, anastamotic leak, bowel obstruction and anesthetic complications. In patients undergoing total gastrectomy with reconstruction for cancer, morbidity has been as high as 60% and mortality ranges from 0-4% (24). However studies, albeit small numbers, looking specifically at PTG have shown that the surgery can be done with low morbidity and no mortality (8,20,22).
Several studies have established that many patients experience diarrhea, fatigue, postprandial discomfort, and reflux after total gastrectomy (22,27). Significant reduction in body weight (10-20%) occurs and personal body image is affected. The majority of post-operative gastrointestinal function and QOL following gastrectomy comes from studies involving resection for cancer. This data may not be entirely applicable to PTG patients because of potential impact of an advanced cancer diagnosis on long-term mental QOL and lack of long-term survival in this group. Worster et al.’s prospective cohort study of 32 patients undergoing prophylactic gastrectomy used validated general and specific for post-gastrectomy QOL questionnaires at 1, 3, and 12 months post-surgery and annually thereafter. The study showed an impairment of well-being within 30 days of surgery but mental, physical, and emotional functionality returned to preoperative levels within 6 months to 1 year (22). These findings are also consistent with several other studies (28,29). Although these studies show that QOL related to daily functioning returns to baseline, individual symptoms persist long-term (22). Many of these symptoms are related to dumping syndrome, including diarrhea, fatigue, reflux, and discomfort when eating. In addition to dumping symptoms, eating restrictions, and body image change persist post-surgery. Based on this study global QOL appears to fully recovery, however there remain some specific aspects of both physical and mental health that persist long term.
Summary
HDGC represents 1-3% of familial gastric cancers. Screening for families with gastric and LBC should occur to determine who should be tested for CDH1 mutations. Endoscopy is not a recommended screening modality as it is not sensitive enough to identify early cancers. The treatment of choice for HDGC is PTG with D1 node dissection and appropriate reconstruction, beginning at age 20, or 5 years younger than the age of the earliest familial diagnosis. Women should undergo annual mammogram and breast MRI screening at a young age. QOL after PTG is diminished within the first postoperative month but returns to baseline within 1 year compared to the general public.
Familial adenomatous polyposis (FAP)
FAP is characterized by the development of multiple (>100) colorectal adenomas throughout the colon and rectum (30). Because of the high lifetime risk of developing cancer and the high penetrance of FAP, strict guidelines for diagnosis and screening have been meticulously developed to identify those who may harbor the disease. Consequently, there are well-established regimens for surgical prophylaxis. The role of the surgeon in caring for these patients involves consideration for prophylactic surgery, endoscopic surveillance, and the possibility of additional surgery if cancer develops in remaining at risk tissue. FAP and AFAP also have a propensity for developing upper gastrointestinal neoplasms and extra-intestinal pathologic conditions. Duodenal and peri-ampullary adenomas may affect up to 90% of patients with FAP, and have a 10% lifetime risk of developing carcinoma proximal to the ligament of Treitz (31-33). Fundic gland gastric polyps can also complicate FAP, but pose minimal risk of carcinoma (34). Desmoid tumors occur in 15 to 20 percent of patients with FAP, causing significant morbidity via obstruction or encroachment on mesenteric vessels (35-37). Papillary and follicular thyroid cancer and pediatric hepatoblastoma have a known association with FAP (38).
Epidemiology
Identification of the APC gene mutation is paramount in these patients due to the autosomal dominant transmission, and a high penetrance with a cancer risk approaching 100% by age 60 (30,39,40). The mutation is responsible for 1% of colon cancers in the United States, and occurs once in every 10,000 to 30,000 births (41). The average age of cancer diagnosis is 40, with a large portion of those with the mutation developing adenomatous polyps by age 35 (40). There is no gender-specific or geographic disposition. These patients are also at risk of developing peri-ampullary and duodenal adenomas, as well as desmoid tumors (32,42,43). AFAP has a similarly high lifetime risk of developing CRC upwards of 80% but has a later mean age of diagnosis and onset of disease at 56 (44,45).
Pathophysiology
The APC gene is a tumor suppressor gene located on chromosome 5q21 and 5q22. In FAP it is inactivated resulting in loss of function (30,40,44,46). The mutation is inherited via autosomal dominant distribution and has variable but typically high penetrance. Some patients have no family history of FAP and approximately 25 percent of mutations are de novo (46). Analysis has shown over 800 different pathogenic mutations in APC, and analysis of disease phenotype has revealed correlations with the site of the mutation for numbers of polyps, age of onset and extra-intestinal manifestations (30,47). This allows clinically helpful predictions of the course of the disease and timing of surgery. The classically known FAP mutations are found between codons 169 to 1,393. AFAP patients will have mutations of the gene at the terminal ends: 5’ to codon 158 and 3’ to codon 1,596 (44-46). The loss of function propagates the dysplasia-carcinoma sequence throughout the entire colon and rectum, resulting in polyposis.
Diagnosis
The diagnosis of FAP is usually based on the clinical finding of 100 or more polyps, or with fewer than 100 polyps but with a family history of FAP (30). AFAP is phenotypically expressed as 10-99 polyps with a right-sided predominance (30,40,48). Genetic testing should be performed for confirmation of the diagnosis of FAP in a patient with colon polyps or prior to development of polyps in patients who are at risk for FAP. First-degree relatives of FAP patients should undergo genetic counseling and screening for FAP between the ages of 10 and 12 years of age (30). The diagnosis of FAP is made via genetic testing and appropriate screening in those with a family history of FAP or AFAP. For de novo mutations, the diagnosis is typically made during endoscopic examination. The typical diagnostic procedure of choice in detection of CRC is endoscopy. The hallmark of FAP is hundreds to thousands of pan-colonic adenomatous polyps. Histologically, these polyps pose no greater risk of colorectal carcinoma in patients who have adenomas without FAP. The quantity of polyps numbering in the hundreds to thousands is what leads to the inevitable malignant transformation to CRC.
Treatment
Treatment strategies should be determined on the basis of disease presence (polyps), family history/mutation location, and life circumstances. Patients with a personal history of classical FAP should undergo prophylactic proctocolectomy or colectomy at a determined appropriate time. Patients without polyps but with a known APC disease-causing mutation should be recommended for flexible endoscopy every 12 months beginning at 10-15 years of age. Patients without polyps and negative genetic testing results but with family history of mutation can undergo colorectal screening based on average risk recommendations. Patients without polyps, with a family history of mutation but decline testing should have endoscopy beginning at 10-15 years of age and gradually decrease the interval over time if clinical disease is not found (49). CRC is rare before the age of 20 so prophylactic surgeries can usually be delayed until physical maturity, particularly in asymptomatic patients (39). Beyond that, when to perform the surgery is important for the patient from a social and lifestyle perspective balanced against risk of malignancy. Patients who develop symptoms such as bleeding or obstruction should have surgical intervention at the time of presentation given a high risk of malignancy (50). Timing of surgery for female FAP patients involves fertility and timing of childbirth considerations. Extensive pelvic surgeries can compromise fecundity through scarring, pelvic nerve injuries and damage to pelvic structures (39,51). Often the patients will choose a time in life that is ideal for surgery and postoperative recovery, such as a period between jobs or a transition period before or after higher education (39).
The surgical prophylactic treatment of FAP has three utilized procedures: total proctocolectomy with end ileostomy, total proctocolectomy with ileal-pouch anal anastomosis (IPAA), and total colectomy abdominal colectomy with ileorectal anastomosis (IRA) (47). Total proctocolectomy with end ileostomy has become less common given most patients desire to retain intestinal continuity. The chosen procedure is a balance of reducing future cancer risk while preserving bowel function and QOL. While IRA maintains better bowel function, there is cancer risk associated with the remaining rectum. IPAA reduces the CRC risk to a very small level, but results in worse bowel function when compared to an IRA (39). Studies consistently show better bowel function for IRA when compared to IPAA particularly in regard to nocturnal soiling, incontinence and frequency of nighttime bowel movements (52).
Historically the need for completion proctectomy following colectomy with IRA could exceed 30% due to the development of rectal cancer. However, the advent of IPAA allows patients who are at high risk for rectal cancer but desired to maintain intestinal continuity an option other than IRA. This allows better selection of patients to undergo IRA, thereby decreasing the rate of salvage proctectomy. The Cleveland Clinic examined their experience with IRA before and after the introduction of IPAA. The completion proctectomy rate dropped from 50% in the pre-pouch era to 2% in the pouch era (47). IRA for FAP is the preferred operation if the rectum is relatively spared. Less than five rectal adenomas at initial presentation correlate with a low risk of failure with IRA. Conversely, 20 or more rectal adenomas imply severe disease and IRA should be avoided (53). Given the fewer number of polyps and the later onset, AFAP patients are good candidates for IRA (39). Adenomas and carcinomas can develop in patients following IPAA for FAP. This can occur in the transitional zone of the anus or the ileal pouch (39,54). Finally, IPAA is still possible with comparable outcomes after an initial IRA (55).
There are two types of anastomosis for IPAA, hand-sewn and stapled. The hand-sewn anastomosis sews the ileum to the dentate line after first removing all of the mucosa above this. This has the advantage of removing theoretically all of the at risk tissue. The stapled technique creates the anastamosis 1-2 cm above the dentate line preserving the transition zone. This has the theoretical advantage of a lower risk of anal sphincter damage given less dissection with an improvement in post-operative bowel function. Furthermore, the transitional zone is implicated in the discrimination between feces and gas. Finally the extra 1-2 cm above the dentate can aid in creating an anastamosis with a short mesentery or other condition limiting length into the pelvis of the ileum (56). There have been a number of prospective, randomized trials comparing a hand-sewn to a stapled anastomosis that have not shown a significant difference in postoperative bowel function (57-60). However, these are small studies and may not be adequately powered to show a difference. To account for this a meta-analysis has been performed to increase the statistical power. The meta-analysis by Schluender et al. included only prospective randomized trials and again found no significant differences in postoperative bowel function but was again limited by small numbers (86 hand-sewn patients compared to 98 stapled patients) (61). A larger meta-analysis that included prospective randomized trials but also prospective non-randomized and retrospective studies evaluated 4,183 patients and found more frequent incontinence to liquid stool and an increase in nighttime seepage in the hand-sewn group. The mean number of stools per day, nighttime bowel movements, and daytime seepage were all similar (62). Finally, a very large single institution prospective database found improved overall QOL, and although the adenoma rate was higher in the stapled group, cancer rates were similar (63). This increased risk of polyps and dysplasia in the stapled group but similar overall rates of cancer has been seen in several other studies as well (64-66). This may be related to an inability to completely eradicate at risk mucosa. Residual rectal mucosa is present in up to 20% of patients after mucosectomy and there is a low rate of dysplasia within the transitional zone (67). Remzi et al. found a 4.5% incidence of anal transitional zone (ATZ) dysplasia at a median follow-up of 130 months and no cases of invasive cancer (66). Overall comparison between the two techniques regarding functional outcome and cancer risk reduction does not show a clear advantage of either and both techniques should be in the skill set of surgeons performing restorative proctocolectomy. All patients regardless of the type of prophylactic surgery performed require surveillance due to the tendency for adenomas to develop in the ileum, as well as in the rectum if IRA is performed (30,68,69).
As mentioned previously, the location of the mutation on the APC gene can impact treatment decisions. Patients with codon 1,309 or 1,328 mutations have onset of polyps in their 20’s and profuse polyposis and should undergo IPAA because of the risk of rectal cancer if IRA is done (39,47). Mutations at the 5’ end and 3’ end of the APC gene are associated with AFAP associated with a later age of onset and fewer polyps and can generally undergo IRA (30,39,47). Although in general there is correlation between a specific genotype and phenotype, there can be marked heterogeneity in expression, between patients with the same mutations (30). The decision about which operation to recommend is driven primarily by phenotype (polyposis severity).
Mutations 3’ of codon 1,400 are associated with desmoid disease. Desmoid disease can lead to serious morbidity and potential mortality in patients with FAP and a strategy is to defer surgery in these patients for as long as possible may be appropriate (47). Patients predisposed to desmoid tumors also prove to be challenging when planning surgery, as they tend to form even after the colon is removed, resulting in extrinsic compression of peritoneal structures and organs (42,43,70,71). In this subset of patients who develop desmoids, IPAA is preferred; additional surgery or completion proctectomy would be extremely challenging (70,71). In IRA patizents, if desmoids do develop and propagate adhesions and mass effect, completion proctectomy may become extremely problematic (37,38).
Surveillance
Surveillance is dependent on the initial prophylactic surgery chosen by the surgeon and the patient. All patients require surveillance due to the propensity for ileal adenomas to develop postoperatively. At 7 years follow-up in IPAA patients, there is a 42% risk of pouch polyposis (72). In patients who have undergone IRA, annual endoscopic surveillance and detection of polyps dictates future surgical intervention. If greater than 20 polyps are identified, completion proctectomy is recommended (39,53).
In FAP patients who have undergone prophylactic surgery, the most common cause of death is peri-ampullary and duodenal adenocarcinoma. The American Society for Gastrointestinal Endoscopy recommendations includes front and side viewing endoscopic examinations beginning at the age of lower endoscopic screening, however there is no established frequency or interval of endoscopic examination (68). European guidelines use Spigelman classification (Table 1) of polyp burden to dictate interval of endoscopy (38,73). Pancreas-preserving total duodenectomies have been performed for large polyp burdens with good results. Other authors report performing pylorus-preserving pancreaticoduodenectomy and total duodenectomy without pancreatectomy but no standard of case for prophylactic upper gastrointestinal surgery has been established (32,74-78).
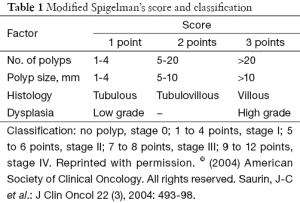
Full table
Complications and QOL
A meta-analysis of 12 studies with IRA versus IPAA had comparable postoperative complication rates, including bleeding, sepsis, anastomotic leak, and obstruction, but IPAA returned to the operating room for correction within 30 days more frequently (69). There is decreased fertility in females and impotence in males following IPAA that is not observed in IRA (51,79). Although studies uniformly show differences in post-operative bowel function between IRA and IPAA, differences in overall QOL are not identified in all studies (52,80-85).
The lack of an impact of altered bowel function on overall QOL is seen also following IPAA. A recent evaluation of QOL following IPAA in 116 patients with FAP showed that almost half of the patients reported greater than six bowel movements per day, 29% reported at least two nocturnal bowel movements, one quarter had impaired continence, 47% complained of daytime seepage and 57% with nighttime seepage. Despite these significant changes in bowel function their generic QOL scores were similar to the general population (86). It appears that assessing patients’ QOL with generic measures is insufficient to evaluate the impact of surgery on their lives (87). Evaluations into the impact of surgery may need to focus on more specific gastrointestinal functional concerns to adequately council patients regarding options.
Summary
FAP is the most extensively studied genetic syndrome with indications for prophylactic surgery. Several methods of treatment have been established, each with its own advantages and disadvantages. Those who possess the APC gene mutation or fall into the category of AFAP should undergo prophylactic surgery. The extent and specific procedure should be dictated by disease phenotype and patient preferences. Surveillance is a necessity to assess for future adenomas, and upper endoscopy is also warranted to detect duodenal and peri-ampullary adenomas. Patients do experience increased frequency of stools compared to the general public, as well as night soiling and incontinence, but objective measurements of their QOL are equivalent to the general public.
Hereditary nonpolyposis colon cancer
Hereditary nonpolyposis colorectal cancer (HNPCC) or LS is an autosomal dominant condition caused by a mutation in one of the mismatch repair (MMR) genes: MLH1, MSH2, MSH6 or PMS2 (88). The primary predisposition for cancer in these patients is right-sided colon cancer and endometrial cancer. Additional associated malignancies include stomach, breast, ovaries, urinary tract, brain, and soft tissues (89,90). HNPCC is probably the most common inherited CRC syndrome accounting for 1-3% of all CRC (88). Prophylactic surgery for HNPCC differs to that of FAP in terms of extent of colectomy. The role for prophylactic surgery in HNPCC is not as well defined as in FAP.
Epidemiology
MLH1 and MSH2 are the most commonly associated mutations in the MMR genes, present in 90% of HNPCC patients (91). The overall spectrum of HNPCC has a 70% risk of developing cancer by age 70 and a mean age of diagnosis in the fifth decade of life (89). CRC is predominantly right sided, but the risk of metachronous cancers is relatively high in the remainder of the colon, with a 40% risk of developing a second tumor within 7 years. There is wide variation in cancer risk within and between families indicating that the risk is influenced by environmental and genetic factors (92). Women also have a high lifetime risk of developing endometrial cancer at 40-60%, with mean age of diagnosis at 50, and a 12% lifetime risk of ovarian cancer (93,94).
Pathophysiology
The DNA MMR genes MSH2, MLH1, MSH6, PMS1, PMS2, and MLH3 have all been identified in the involvement of HNPCC, with a lifetime cancer risk of approximately 70-80% (95,96). These genes, when mutated or silenced via hypermethylation, allow damaged DNA to replicate indiscriminately, resulting in transformation and carcinogenesis (97). Extensive modification via microsatellite instability is thought to be responsible for adenocarcinoma present in HNPCC patients and 15% of other CRCs (98).
Diagnosis
HNPCC is suspected in families with early onset of CRC or associated malignancies (endometrium, renal pelvis/ureter, stomach, small bowel, ovary, brain, and also sebaceous tumors), a high rate of synchronous and metacronous cancers and a right sided predominance of the colon cancers (99). Multiple diagnostic guidelines to identify these individuals have been developed, the most common being Amsterdam and Bethesda criteria (100) (Tables 2,3). However these criteria are complex and lack sensitivity (88). The population most easily identified is those who present with CRC. A prospective molecular screening was applied to over 10,000 CRC cases and found an incidence of HNPCC associated mutations of 3.1% (101). This has led some groups to recommend testing all CRC tumors with immunohistochemistry or microsattelite instability (MSI) (88). This approach has been shown to be cost-effective (102). Patients with MSI-high tumors should undergo testing for germline MMR mutations (100).

Full table

Full table
Treatment
There are currently no guidelines for prophylactic surgery in asymptomatic patients with HNPCC (96). There are several concerns regarding making recommendations for prophylactic colon surgery including: a high risk of developing several types of cancer (not just colon), risk of cancer in retained rectum, timing of surgery (HNPCC is not characterized by a large number of polyps), and because not all mutation carriers will develop cancer (103). The argument for prophylactic colectomy in HNPCC is the risk of developing colon cancer in HNPCC is not significantly lower than in FAP. The penetrance of FAP is near 100% and the penetrance in HNPCC is 70-85% and the average age of onset of cancer is less than a decade later in HNPCC. Additionally because of the right-sided predominance of cancer in HNPCC, IRA is the procedure of choice instead of IPAA (104,105). IRA is also amenable to minimally invasive techniques potentially further decreasing impact of surgery on QOL (103,105).
There are no direct comparisons between surveillance and prophylactic surgery in HNPCC. Syngal et al. used a Mrakov model to evaluate the benefit of surveillance colonoscopy to prophylactic total colectomy to total proctocolectomy at various ages. They found that total protocolectomy at age 25 years had an increase in life expectancy (LE) of 15.6 years compared to subtotal colectomy of 15.3 years and surveillance colonoscopy of 14.2 years. They also found that the benefit of surgery over colonoscopy becomes less than 1 year if surgery is done at an age of 40 or greater and negligible if done following the diagnosis of a cancer. Finally, if quality adjusted LE is examined, surveillance is superior to surgery (106). Given the available evidence, prophylactic colorectal surgery can be considered in select individuals based upon age, the histologic characteristics of the polyp(s) if present, cancer pattern in the family and probably most importantly their willingness and ability to follow the recommended rigorous surveillance program (105,107).
Prophylactic surgery for endometrial and ovarian cancer has been better established in HNPCC. There is sufficient evidence supporting prophylactic hysterectomy and oophorectomy; a cohort of 61 patients who underwent prophylactic surgery had no evidence of cancer after 10-year follow-up (108). Evidence supports the use of prophylactic gynecologic surgery in this patient population and should be routinely offered (109). Laparoscopic colectomy, hysterectomy, and bilateral oophorectomy can all be performed within the same operation safely (103).
Surveillance
Colonoscopy should be performed at regular intervals in patients with HNPCC beginning at 20-25 years. Colonoscopy every 3 years has been shown to reduce CRC and decrease overall mortality by 65% in HNPCC cohorts (110). Several studies have looked prospectively at shorter intervals to decrease the development of interval cancers. It appears that decreasing the interval to 1-2 years is optimal to prevent advanced stage interval cancers (88,100).
Screening for endometrial cancer is also important in HNPCC. The American Cancer Society recommends annual trans-vaginal ultrasound and endometrial sampling annually starting at age 30 in patients with LS (111).
Quality of life (QOL)
QOL scores for IPAA and IRA have been demonstrated in patients with FAP above. For patients with LS who undergo similar procedures, QOL can be assumed equivalent. Evidence supports the laparoscopic approach for prophylactic surgery. Consistent with most laparoscopic procedures decreased postoperative pain and recovery time is well described in the literature (103,105).
Summary
HNPCC is genetically and biologically different from FAP, with a more diverse spectrum of disease phenotype. Identification of asymptomatic patients can be difficult given current criteria that lack sensitivity. Screening all CRC cancer patients’ tumors offers a potential to identify index cases and additional family members. The role of prophylactic colon surgery is not well defined but can be considered in select individuals. Females should be routinely screened for endometrial cancer and prophylactic hysterectomy can be offered if the patient does not desire children.
MUTYH and hamartomatous polyposis syndromes
Other less common genetic syndromes that are inherited differently include MUTY homolog associated polyposis (MAP), PJS, and Juvenile polyposis syndrome (JPS).
MYH is a base excision repair (BER) gene located on chromosome 1p35 and is involved in repairing oxidative DNA damage (112). MUTYH is a germline mutation with widely varying phenotype, ranging from classic polyposis with adenomas in the thousands, to a similar presentation of AFAP, with only several to dozens of polyps; and polyps can be serrated. Given the clinical variability it has been proposed that MUTYH testing should be considered in early-onset CRC patients with intact DNA MMR, regardless of family history or number of colonic polyps.
In a recent population based case-control study MUTYH mutations accounted for 0.7% of CRC (113). MUTYH-related CRC are seen in younger patients and have a right-sided predominance. The risk of CRC is highest in patients who are homozygous for the mutation but there appears to be a small increase in risk for even heterozygotes. Biallelic CRC risk approaches 80% by age 70 (114). Associated extra-intestinal manifestations of the syndrome include duodenal adenomas, skin, ovarian, and bladder (115). There are no specific screening guidelines for MUTYH mutations established. However, given the established increased CRC risks guidelines for FAP can be used but beginning at a later age (25-30 years) given later onset (112). There are no established guidelines regarding prophylactic surgery but depending on number and location of the polyps, patients with MYH biallelic mutations should undergo total colectomy or restorative proctocolectomy, just like AFAP (116). Extensive polyposis involving the rectum mandates proctocolectomy (117). If the rectum is spared they can probably undergo IRA but long-term data regarding subsequent rectal cancers in the remaining rectum is limited (38).
PJS is an autosomal dominant inherited syndrome that predisposes the affected individual to a number of malignancies. The mutation is in the serine threonine kinase (STK) gene (115). PJS, patients present with buccal and mucosal pigmentation as well as gastrointestinal hamartomatous polyps (118). The diagnosis of PJS requires histologic confirmation of a hamartomatous, Peutz-Jeghers-type polyp. These polyps can also be seen in individuals who do not have PJS; therefore in addition to the hamartoma at least two of the following must be present: small bowel polyposis, family history of PJS; or pigmented macules of buccal mucosa, lips, fingers, and toes (119). These hamartomas cause a number of issues for the patients, including intussusception, bowel obstruction, and dysplastic transformation to adenocarcinoma (118). In addition to an increase risk of CRC other associate cancers include gastric, small bowel, pancreatic, breast, ovarian, lung, cervical, and uterine/testicular (120). A European consensus statement recommended upper endoscopy, colonoscopy and capsule endoscopy beginning at age 8 and repeated every 3 years if polyps present. If no polyps are present the exams are repeated at age 18 and then every 3 years. At age 50 surveillance should be every 1-2 years (121). Although the risk of all cause cancer death is elevated in PJS the risk of CRC is only 3-8% in followed cohorts (118). Therefore prophylactic colectomy cannot be advocated based on the available evidence.
Conclusions
As research and technology advances, these congenital and inherited conditions may one day be treated via gene therapy or cellular manipulation. For now, prophylactic surgery is the only curative option. As the surgeon approaches the patient with said condition, he or she must take several things into consideration: the patient’s desire for surgery, their willingness for postoperative surveillance, their postoperative QOL, and genetic testing for their family members who also would need prophylactic surgery. Surgical technique has evolved rapidly over the last few decades and utilization of minimally invasive and laparoscopic techniques has improved patient satisfaction and improved QOL. This can potentially make prophylactic surgery more appealing. Postoperative surveillance methods for recurrence as well as secondary malignancies have improved as the general understanding of the disease process becomes more apparent. The timeline for genetic solutions is unknown at this time; thus, the surgeon remains an important part of preventing the development of cancer in patients with inherited cancer syndromes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Coviello LC, Wascher RA. Hereditary neoplasia syndromes and the role of the surgeon. Fam Cancer 2008;7:97-102. [PubMed]
- Nagy R, Sweet K, Eng C. Highly penetrant hereditary cancer syndromes. Oncogene 2004;23:6445-70. [PubMed]
- Corso G, Figueiredo J, Biffi R, et al. E-cadherin germline mutation carriers: clinical management and genetic implications. Cancer Metastasis Rev 2014;33:1081-94. [PubMed]
- Ziogas D, Roukos DH. CDH1 testing: can it predict the prophylactic or therapeutic nature of total gastrectomy in hereditary diffuse gastric cancer? Ann Surg Oncol 2009;16:2678-81. [PubMed]
- Roukos DH. Genome-wide association studies and aggressive surgery toward individualized prevention, and improved local control and overall survival for gastric cancer. Ann Surg Oncol 2009;16:795-8. [PubMed]
- Dixon M, Seevaratnam R, Wirtzfeld D, et al. A RAND/UCLA appropriateness study of the management of familial gastric cancer. Ann Surg Oncol 2013;20:533-41. [PubMed]
- Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature 1998;392:402-5. [PubMed]
- Chen Y, Kingham K, Ford JM, et al. A prospective study of total gastrectomy for CDH1-positive hereditary diffuse gastric cancer. Ann Surg Oncol 2011;18:2594-8. [PubMed]
- Pharoah PD, Guilford P, Caldas C, et al. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001;121:1348-53. [PubMed]
- Fitzgerald RC, Hardwick R, Huntsman D, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet 2010;47:436-44. [PubMed]
- Humar B, Guilford P. Hereditary diffuse gastric cancer: a manifestation of lost cell polarity. Cancer Sci 2009;100:1151-7. [PubMed]
- Brooks-Wilson AR, Kaurah P, Suriano G, et al. Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet 2004;41:508-17. [PubMed]
- Yamada H, Shinmura K, Ito H, et al. Germline alterations in the CDH1 gene in familial gastric cancer in the Japanese population. Cancer Sci 2011;102:1782-8. [PubMed]
- Graziano F, Humar B, Guilford P. The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice. Ann Oncol 2003;14:1705-13. [PubMed]
- Blair V, Martin I, Shaw D, et al. Hereditary diffuse gastric cancer: diagnosis and management. Clin Gastroenterol Hepatol 2006;4:262-75. [PubMed]
- Caldas C, Carneiro F, Lynch HT, et al. Familial gastric cancer: overview and guidelines for management. J Med Genet 1999;36:873-80. [PubMed]
- Park JG, Yang HK, Kim WH, et al. Report on the first meeting of the International Collaborative Group on Hereditary Gastric Cancer. J Natl Cancer Inst 2000;92:1781-2. [PubMed]
- Hebbard PC, Macmillan A, Huntsman D, et al. Prophylactic total gastrectomy (PTG) for hereditary diffuse gastric cancer (HDGC): the Newfoundland experience with 23 patients. Ann Surg Oncol 2009;16:1890-5. [PubMed]
- Shaw D, Blair V, Framp A, et al. Chromoendoscopic surveillance in hereditary diffuse gastric cancer: an alternative to prophylactic gastrectomy? Gut 2005;54:461-8. [PubMed]
- Norton JA, Ham CM, Van Dam J, et al. CDH1 truncating mutations in the E-cadherin gene: an indication for total gastrectomy to treat hereditary diffuse gastric cancer. Ann Surg 2007;245:873-9. [PubMed]
- Fujita H, Lennerz JK, Chung DC, et al. Endoscopic surveillance of patients with hereditary diffuse gastric cancer: biopsy recommendations after topographic distribution of cancer foci in a series of 10 CDH1-mutated gastrectomies. Am J Surg Pathol 2012;36:1709-17. [PubMed]
- Worster E, Liu X, Richardson S, et al. The impact of prophylactic total gastrectomy on health-related quality of life: a prospective cohort study. Ann Surg 2014;260:87-93. [PubMed]
- Cisco RM, Norton JA. Hereditary diffuse gastric cancer: surgery, surveillance and unanswered questions. Future Oncol 2008;4:553-9. [PubMed]
- Lynch HT, Silva E, Wirtzfeld D, et al. Hereditary diffuse gastric cancer: prophylactic surgical oncology implications. Surg Clin North Am 2008;88:759-78. vi-vii. [PubMed]
- Rogers WM, Dobo E, Norton JA, et al. Risk-reducing total gastrectomy for germline mutations in E-cadherin (CDH1): pathologic findings with clinical implications. Am J Surg Pathol 2008;32:799-809. [PubMed]
- Nozaki I, Nasu J, Kubo Y, et al. Risk factors for metachronous gastric cancer in the remnant stomach after early cancer surgery. World J Surg 2010;34:1548-54. [PubMed]
- Shenoy S, Palmer C, Dunsworth T, et al. Hereditary diffuse gastric cancer: genetics, prophylactic total gastrectomy, and follow up. Gastrointestinal Cancer: Targets and Therapy 2011;1:15-9.
- Wu CW, Chiou JM, Ko FS, et al. Quality of life after curative gastrectomy for gastric cancer in a randomised controlled trial. Br J Cancer 2008;98:54-9. [PubMed]
- Hackenson D, Edelman DA, McGuire T, et al. Prophylactic laparoscopic gastrectomy for hereditary diffuse gastric cancer: a case series in a single family. JSLS 2010;14:348-52. [PubMed]
- Aihara H, Kumar N, Thompson CC. Diagnosis, surveillance, and treatment strategies for familial adenomatous polyposis: rationale and update. Eur J Gastroenterol Hepatol 2014;26:255-62. [PubMed]
- Björk J, Akerbrant H, Iselius L, et al. Periampullary adenomas and adenocarcinomas in familial adenomatous polyposis: cumulative risks and APC gene mutations. Gastroenterology 2001;121:1127-35. [PubMed]
- Benetatos N, Ammori MB, Ammori BJ. Laparoscopic pancreas-preserving total duodenectomy for familial adenomatous polyposis. Surg Laparosc Endosc Percutan Tech 2011;21:e332-5. [PubMed]
- Ma T, Jang EJ, Zukerberg LR, et al. Recurrences are common after endoscopic ampullectomy for adenoma in the familial adenomatous polyposis (FAP) syndrome. Surg Endosc 2014;28:2349-56. [PubMed]
- Bianchi LK, Burke CA, Bennett AE, et al. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008;6:180-5. [PubMed]
- Clark SK, Neale KF, Landgrebe JC, et al. Desmoid tumours complicating familial adenomatous polyposis. Br J Surg 1999;86:1185-9. [PubMed]
- Bertario L, Russo A, Sala P, et al. Genotype and phenotype factors as determinants of desmoid tumors in patients with familial adenomatous polyposis. Int J Cancer 2001;95:102-7. [PubMed]
- Nieuwenhuis MH, Lefevre JH, Bülow S, et al. Family history, surgery, and APC mutation are risk factors for desmoid tumors in familial adenomatous polyposis: an international cohort study. Dis Colon Rectum 2011;54:1229-34. [PubMed]
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008;57:704-13. [PubMed]
- Warrier SK, Kalady MF. Familial adenomatous polyposis: challenges and pitfalls of surgical treatment. Clin Colon Rectal Surg 2012;25:83-9. [PubMed]
- Petersen GM, Slack J, Nakamura Y. Screening guidelines and premorbid diagnosis of familial adenomatous polyposis using linkage. Gastroenterology 1991;100:1658-64. [PubMed]
- Wennstrom J, Pierce ER, McKusick VA. Hereditary benign and malignant lesions of the large bowel. Cancer 1974;34 suppl:850-7. [PubMed]
- Church J, Simmang C. Standards Task Force, et al. Practice parameters for the treatment of patients with dominantly inherited colorectal cancer (familial adenomatous polyposis and hereditary nonpolyposis colorectal cancer). Dis Colon Rectum 2003;46:1001-12. [PubMed]
- Vitellaro M, Bonfanti G, Sala P, et al. Laparoscopic colectomy and restorative proctocolectomy for familial adenomatous polyposis. Surg Endosc 2011;25:1866-75. [PubMed]
- Giardiello FM, Brensinger JD, Luce MC, et al. Phenotypic expression of disease in families that have mutations in the 5' region of the adenomatous polyposis coli gene. Ann Intern Med 1997;126:514-9. [PubMed]
- Hernegger GS, Moore HG, Guillem JG. Attenuated familial adenomatous polyposis: an evolving and poorly understood entity. Dis Colon Rectum 2002;45:127-34; discussion 134-6. [PubMed]
- Burt RW, DiSario JA, Cannon-Albright L. Genetics of colon cancer: impact of inheritance on colon cancer risk. Annu Rev Med 1995;46:371-9. [PubMed]
- da Luz Moreira A, Church JM, Burke CA. The evolution of prophylactic colorectal surgery for familial adenomatous polyposis. Dis Colon Rectum 2009;52:1481-6. [PubMed]
- Lynch HT, Smyrk T, McGinn T, et al. Attenuated familial adenomatous polyposis (AFAP). A phenotypically and genotypically distinctive variant of FAP. Cancer 1995;76:2427-33. [PubMed]
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 1997;112:594-642. [PubMed]
- Bülow S, Bülow C, Nielsen TF, et al. Centralized registration, prophylactic examination, and treatment results in improved prognosis in familial adenomatous polyposis. Results from the Danish Polyposis Register. Scand J Gastroenterol 1995;30:989-93. [PubMed]
- Slors FJ, van Zuijlen PP, van Dijk GJ. Sexual and bladder dysfunction after total mesorectal excision for benign diseases. Scand J Gastroenterol Suppl 2000.48-51. [PubMed]
- Günther K, Braunrieder G, Bittorf BR, et al. Patients with familial adenomatous polyposis experience better bowel function and quality of life after ileorectal anastomosis than after ileoanal pouch. Colorectal Dis 2003;5:38-44. [PubMed]
- Church J, Burke C, McGannon E, et al. Predicting polyposis severity by proctoscopy: how reliable is it? Dis Colon Rectum 2001;44:1249-54. [PubMed]
- Church J. Ileoanal pouch neoplasia in familial adenomatous polyposis: an underestimated threat. Dis Colon Rectum 2005;48:1708-13. [PubMed]
- Björk J, Akerbrant H, Iselius L, et al. Outcome of primary and secondary ileal pouch-anal anastomosis and ileorectal anastomosis in patients with familial adenomatous polyposis. Dis Colon Rectum 2001;44:984-92. [PubMed]
- Trigui A, Frikha F, Rejab H, et al. Ileal pouch-anal anastomosis: Points of controversy. J Visc Surg 2014;151:281-8. [PubMed]
- Hallgren T, Fasth S, Nordgren S, et al. The stapled ileal pouch--anal anastomosis. A randomized study comparing two different pouch designs. Scand J Gastroenterol 1990;25:1161-8. [PubMed]
- Luukkonen P, Järvinen H. Stapled vs hand-sutured ileoanal anastomosis in restorative proctocolectomy. A prospective, randomized study. Arch Surg 1993;128:437-40. [PubMed]
- Reilly WT, Pemberton JH, Wolff BG, et al. Randomized prospective trial comparing ileal pouch-anal anastomosis performed by excising the anal mucosa to ileal pouch-anal anastomosis performed by preserving the anal mucosa. Ann Surg 1997;225:666-76; discussion 676-7. [PubMed]
- Choen S, Tsunoda A, Nicholls RJ. Prospective randomized trial comparing anal function after hand sewn ileoanal anastomosis with mucosectomy versus stapled ileoanal anastomosis without mucosectomy in restorative proctocolectomy. Br J Surg 1991;78:430-4. [PubMed]
- Schluender SJ, Mei L, Yang H, et al. Can a meta-analysis answer the question: is mucosectomy and handsewn or double-stapled anastomosis better in ileal pouch-anal anastomosis? Am Surg 2006;72:912-6. [PubMed]
- Lovegrove RE, Constantinides VA, Heriot AG, et al. A comparison of hand-sewn versus stapled ileal pouch anal anastomosis (IPAA) following proctocolectomy: a meta-analysis of 4183 patients. Ann Surg 2006;244:18-26. [PubMed]
- Kirat HT, Remzi FH, Kiran RP, et al. Comparison of outcomes after hand-sewn versus stapled ileal pouch-anal anastomosis in 3,109 patients. Surgery 2009;146:723-9; discussion 729-30. [PubMed]
- van Duijvendijk P, Vasen HF, Bertario L, et al. Cumulative risk of developing polyps or malignancy at the ileal pouch-anal anastomosis in patients with familial adenomatous polyposis. J Gastrointest Surg 1999;3:325-30. [PubMed]
- von Roon AC, Will OC, Man RF, et al. Mucosectomy with handsewn anastomosis reduces the risk of adenoma formation in the anorectal segment after restorative proctocolectomy for familial adenomatous polyposis. Ann Surg 2011;253:314-7. [PubMed]
- Remzi FH, Fazio VW, Delaney CP, et al. Dysplasia of the anal transitional zone after ileal pouch-anal anastomosis: results of prospective evaluation after a minimum of ten years. Dis Colon Rectum 2003;46:6-13. [PubMed]
- O'Connell PR, Pemberton JH, Weiland LH, et al. Does rectal mucosa regenerate after ileoanal anastomosis? Dis Colon Rectum 1987;30:1-5. [PubMed]
- Hirota WK, Zuckerman MJ, Adler DG, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc 2006;63:570-80. [PubMed]
- Aziz O, Athanasiou T, Fazio VW, et al. Meta-analysis of observational studies of ileorectal versus ileal pouch-anal anastomosis for familial adenomatous polyposis. Br J Surg 2006;93:407-17. [PubMed]
- Durno C, Monga N, Bapat B, et al. Does early colectomy increase desmoid risk in familial adenomatous polyposis? Clin Gastroenterol Hepatol 2007;5:1190-4. [PubMed]
- Sturt NJ, Clark SK. Current ideas in desmoid tumours. Fam Cancer 2006;5:275-85; discussion 287-8. [PubMed]
- Wu JS, McGannon EA, Church JM. Incidence of neoplastic polyps in the ileal pouch of patients with familial adenomatous polyposis after restorative proctocolectomy. Dis Colon Rectum 1998;41:552-6; discussion 556-7. [PubMed]
- Saurin JC, Gutknecht C, Napoleon B, et al. Surveillance of duodenal adenomas in familial adenomatous polyposis reveals high cumulative risk of advanced disease. J Clin Oncol 2004;22:493-8. [PubMed]
- Ruo L, Coit DG, Brennan MF, et al. Long-term follow-up of patients with familial adenomatous polyposis undergoing pancreaticoduodenal surgery. J Gastrointest Surg 2002;6:671-5. [PubMed]
- Gallagher MC, Shankar A, Groves CJ, et al. Pylorus-preserving pancreaticoduodenectomy for advanced duodenal disease in familial adenomatous polyposis. Br J Surg 2004;91:1157-64. [PubMed]
- Causeret S, François Y, Griot JB, et al. Prophylactic pancreaticoduodenectomy for premalignant duodenal polyposis in familial adenomatous polyposis. Int J Colorectal Dis 1998;13:39-42. [PubMed]
- Gallagher MC, Phillips RK, Bulow S. Surveillance and management of upper gastrointestinal disease in Familial Adenomatous Polyposis. Fam Cancer 2006;5:263-73. [PubMed]
- Delpero JR, Turrini O, Ewald J. Total duodenectomy without pancreatectomy for familial adenomatous polyposis (with video). J Visc Surg 2014;151:473-4. [PubMed]
- Olsen KØ, Juul S, Bülow S, et al. Female fecundity before and after operation for familial adenomatous polyposis. Br J Surg 2003;90:227-31. [PubMed]
- Ambroze WL Jr, Dozois RR, Pemberton JH, et al. Familial adenomatous polyposis: results following ileal pouch-anal anastomosis and ileorectostomy. Dis Colon Rectum 1992;35:12-5. [PubMed]
- Madden MV, Neale KF, Nicholls RJ, et al. Comparison of morbidity and function after colectomy with ileorectal anastomosis or restorative proctocolectomy for familial adenomatous polyposis. Br J Surg 1991;78:789-92. [PubMed]
- Tonelli F, Valanzano R, Monaci I, et al. Restorative proctocolectomy or rectum-preserving surgery in patients with familial adenomatous polyposis: results of a pr [PubMed]
- Soravia C, Klein L, Berk T, et al. Comparison of ileal pouch-anal anastomosis and ileorectal anastomosis in patients with familial adenomatous polyposis. Dis Colon Rectum 1999;42:1028-33; discussion 1033-4. [PubMed]
- Van Duijvendijk P, Slors JF, Taat CW, et al. Quality of life after total colectomy with ileorectal anastomosis or proctocolectomy and ileal pouch-anal anastomosis for familial adenomatous polyposis. Br J Surg 2000;87:590-6. [PubMed]
- Ko CY, Rusin LC, Schoetz DJ Jr, et al. Does better functional result equate with better quality of life? Implications for surgical treatment in familial adenomatous polyposis. Dis Colon Rectum 2000;43:829-35; discussion 835-7. [PubMed]
- Wolf ND, Kadmon M, Wolf RC, et al. Quality of life after restorative proctocolectomy and ileal pouch-anal anastomosis in patients with familial adenomatous polyposis: a matter of adjustment. Colorectal Dis 2011;13:e358-65. [PubMed]
- Young-Fadok TM, Ko CY, Rusin LC, et al. Invited editorial. Dis Colon Rectum 2000;43:835-7.
- Vasen HF, Blanco I, Aktan-Collan K, et al. Revised guidelines for the clinical management of Lynch syndrome (HNPCC): recommendations by a group of European experts. Gut 2013;62:812-23. [PubMed]
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919-32. [PubMed]
- Watson P, Lynch HT. The tumor spectrum in HNPCC. Anticancer Res 1994;14:1635-9. [PubMed]
- Baudhuin LM, Burgart LJ, Leontovich O, et al. Use of microsatellite instability and immunohistochemistry testing for the identification of individuals at risk for Lynch syndrome. Fam Cancer 2005;4:255-65. [PubMed]
- Dunlop MG, Tenesa A, Farrington SM, et al. Cumulative impact of common genetic variants and other risk factors on colorectal cancer risk in 42,103 individuals. Gut 2013;62:871-81. [PubMed]
- Celentano V, Luglio G, Antonelli G, et al. Prophylactic surgery in Lynch syndrome. Tech Coloproctol 2011;15:129-34. [PubMed]
- Aarnio M, Sankila R, Pukkala E, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer 1999;81:214-8. [PubMed]
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 2009;22:191-7. [PubMed]
- Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 2006;296:1507-17. [PubMed]
- Cunningham JM, Christensen ER, Tester DJ, et al. Hypermethylation of the hMLH1 promoter in colon cancer with microsatellite instability. Cancer Res 1998;58:3455-60. [PubMed]
- Jass JR, Cottier DS, Jeevaratnam P, et al. Diagnostic use of microsatellite instability in hereditary non-polyposis colorectal cancer. Lancet 1995;346:1200-1. [PubMed]
- Vasen HF, Watson P, Mecklin JP, et al. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999;116:1453-6. [PubMed]
- Guillem JG, Wood WC, Moley JF, et al. ASCO/SSO review of current role of risk-reducing surgery in common hereditary cancer syndromes. J Clin Oncol 2006;24:4642-60. [PubMed]
- Moreira L, Balaguer F, Lindor N, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA 2012;308:1555-65. [PubMed]
- Mvundura M, Grosse SD, Hampel H, et al. The cost-effectiveness of genetic testing strategies for Lynch syndrome among newly diagnosed patients with colorectal cancer. Genet Med 2010;12:93-104. [PubMed]
- Pocard M, Pomel C, Lasser P. Laparoscopic prophylactic surgery for HNPCC gene mutation carrier: has the time come? Lancet Oncol 2003;4:637-8. [PubMed]
- Church JM, Fazio VW, Lavery IC, et al. Quality of life after prophylactic colectomy and ileorectal anastomosis in patients with familial adenomatous polyposis. Dis Colon Rectum 1996;39:1404-8. [PubMed]
- Lynch HT, Lynch JF, Fitzgibbons R Jr. Role of prophylactic colectomy in Lynch syndrome. Clin Colorectal Cancer 2003;3:99-101. [PubMed]
- Syngal S, Weeks JC, Schrag D, et al. Benefits of colonoscopic surveillance and prophylactic colectomy in patients with hereditary nonpolyposis colorectal cancer mutations. Ann Intern Med 1998;129:787-96. [PubMed]
- Scaife CL, Rodriguez-Bigas MA. Lynch syndrome: implications for the surgeon. Clin Colorectal Cancer 2003;3:92-8. [PubMed]
- Schmeler KM, Lynch HT, Chen LM, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med 2006;354:261-9. [PubMed]
- Meyer LA, Broaddus RR, Lu KH. Endometrial cancer and Lynch syndrome: clinical and pathologic considerations. Cancer Control 2009;16:14-22. [PubMed]
- Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000;118:829-34. [PubMed]
- Smith RA, von Eschenbach AC, Wender R, et al. American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001--testing for early lung cancer detection. CA Cancer J Clin 2001;51:38-75. [PubMed]
- Kastrinos F, Syngal S. Recently identified colon cancer predispositions: MYH and MSH6 mutations. Semin Oncol 2007;34:418-24. [PubMed]
- Cleary SP, Cotterchio M, Jenkins MA, et al. Germline MutY human homologue mutations and colorectal cancer: a multisite case-control study. Gastroenterology 2009;136:1251-60. [PubMed]
- Jenkins MA, Croitoru ME, Monga N, et al. Risk of colorectal cancer in monoallelic and biallelic carriers of MYH mutations: a population-based case-family study. Cancer Epidemiol Biomarkers Prev 2006;15:312-4. [PubMed]
- Patel SG, Ahnen DJ. Familial colon cancer syndromes: an update of a rapidly evolving field. Curr Gastroenterol Rep 2012;14:428-38. [PubMed]
- Lefevre JH, Rodrigue CM, Mourra N, et al. Implication of MYH in colorectal polyposis. Ann Surg 2006;244:874-9; discussion 879-80. [PubMed]
- Nascimbeni R, Pucciarelli S, Di Lorenzo D, et al. Rectum-sparing surgery may be appropriate for biallelic MutYH-associated polyposis. Dis Colon Rectum 2010;53:1670-5. [PubMed]
- Tomlinson IP, Houlston RS. Peutz-Jeghers syndrome. J Med Genet 1997;34:1007-11. [PubMed]
- Giardiello FM, Welsh SB, Hamilton SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med 1987;316:1511-4. [PubMed]
- Hearle N, Schumacher V, Menko FH, et al. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res 2006;12:3209-15. [PubMed]
- Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut 2010;59:975-86. [PubMed]


