The role of Creighton University’s hereditary cancer center
Introduction
Creighton University’s hereditary cancer center (HCC) registry is focused upon the collection and documentation of hereditary cancer families and subsequently identifying and educating patients at high hereditary cancer risk and/or those already affected with cancer regarding their cancer risk based on their position in the family pedigree and/or their genetic mutation status.
We will be using Lynch syndrome (LS) as a model, as it is the most common hereditary colorectal cancer (CRC) syndrome, accounting for 3-5% of the total number of CRC cases (1,2), making the annual estimate for LS-associated CRC in the United States 4,000-6,000, since the estimated CRC in the U.S. for 2015 is 132,700 (3). Those relatives who harbor a deleterious LS mutation can have a lifetime CRC risk of at least 74% (4). Women with LS mutations are at 40-60% lifetime risk of endometrial cancer, with approximately 2% of all endometrial cancer being LS-associated (5). Predisposition also exists for a variety of other cancers, including that of the ovary, stomach, small bowel, pancreato-biliary, upper urinary epithelial tract (uroepithelial), breast, prostate, adrenal cortical, and Muir-Torre syndrome spectrum of skin tumors (sebaceous adenomas, sebaceous carcinomas, keratoacanthomas), as well as brain (glioblastoma) in Turcot’s syndrome (See Table 1).
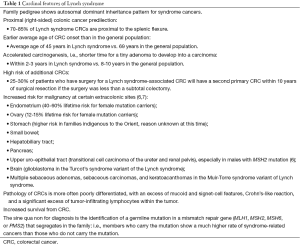
Full table
Other gastrointestinal cancers with significant hereditary components are pancreatic cancer and diffuse gastric cancer. See Table 2 for other hereditary CRC syndromes.

Full table
Collection of detailed and extended family histories is a core function of the HCC registry, accurately documenting the relationships of family members, cancer diagnoses with histological features, age(s) of cancer onset, surveillance and preventive surgical measures, and genetic test results. Accurate documentation of relationships of family members allows for risk assessment calculations for each bloodline family member based upon pedigree position as well as genetic risk based on individual genetic test results. The ability to quickly and accurately calculate pedigree and genetic risk assessments for all bloodline relatives in the family provides the opportunity to inform and educate family members about their cancer risk and recommend personalized screening/prevention plans. In addition, researchers have the ability to quickly identify eligible subjects for a wide variety of research projects. The HCC registry has been an invaluable resource for multiple national and international collaborative studies in the field of cancer genetics, inclusive of the discovery of the LS mismatch repair mutations and hereditary breast-ovarian cancer (HBOC) syndrome BRCA genes.
Mutations in well-characterized genes provide a basis for confirmation of syndrome involvement, personalized management of cancer patients, and predictive testing and management of at-risk relatives (8). While these may be numerically small, study of the genes involved has frequently improved our understanding of pathways involved in nonfamilial cancers (9). Within the families in which these hereditary cancers occur, there exists a tremendous opportunity to achieve early cancer detection and prevention (10-12). Family members identified as carriers of pathogenic LS mutations are provided with screening recommendations along with optional preventive surgical measures, namely risk-reducing hysterectomy and salpingo-oophorectomy in women carriers. These recommendations are provided to family members by trained genetic professionals, knowledgeable in the field of cancer genetics. This service is free of charge to all subjects who enroll in the HCC registry and wish to learn more about their cancer risk, either based on the pedigree alone or when identified through genetic testing.
The HCC registry also has an integral biorepository wherein biological samples have been stored on multiple cancer-affected and unaffected family members over the past 40 years. The foresight to establish cell lines on key family members has proven to be a vital strength of the biorepository in helping families learn of their cancer risk through recent discoveries and genetic testing techniques. Key, informative family members who provided a sample for genetic testing and research who subsequently passed away, had provided their family with a gift, since their sample could be analyzed and tested for genetic mutations discovered long after they had died. Collecting and storing cell lines on various family members throughout multiple generations has also led to insights on the transmission of the genetic mutations and the expressed clinical significance of pre-cancer and cancer phenotypes within each family.
The HCC registry provides a unique setting of involvement in cutting edge research by virtue of the massive collection of LS families with highly detailed data points collected on multiple family members, reaching second- and third-degree relatives and even further for certain families as targeted contacts continue to extend the family history. This unique opportunity allows for HCC researchers to conduct analyses of these families to help determine LS-related risks and associated cancers. In addition, the research conducted within these families in collaboration with cancer genetic researchers around the world allows the transmission of the research findings to the research subjects and family members.
Unfortunately, healthcare providers have difficulty keeping up with new advances in cancer genetics, and commonly are not intimately familiar with the clinical practice guidelines that explicitly include genetic testing, genetic counseling, and appropriate screening measures, with or without informative genetic testing (13). For example, although heredity in certain circumstances poses a striking etiologic factor in a subset of many forms of cancer, in certain families a hereditary diagnosis may be obscured, in part by the rarity of the syndrome, its reduced penetrance, incomplete medical records, and occasionally late age of cancer onset. There is also frequently a lack of glaring phenotypic stigmata of hereditary cancer risk. These factors may obscure a definitive diagnosis, particularly when viewed in concert with cancer’s extensive phenotypic and genotypic heterogeneity. In fact, our experience with many varieties of hereditary cancer has shown similar arrays of confounders.
Clearly, it is clinically imperative to collect data on cancer-prone families, analyze the natural history of their cancers, obtain precious biospecimens when this is appropriate, and utilize cutting edge molecular tools to unravel complexities in the interest of early diagnosis and heightened cancer control. The ultimate goal is based upon an accurate clinical diagnosis, followed by genetic testing of the most pertinent germline mutation that could explain the genesis of the particular hereditary cancer syndrome as evidenced by such germline mutations as BRCA1 and BRCA2 in the HBOC syndrome and the mismatch repair germline mutations (MLH1, MSH2, MSH6, PMS2, EPCAM) in LS. This, of course, will be provided in accord with patients’ informed consent for testing (Table 3). It will be absolutely essential that patients, once they receive results of the testing, receive a full explanation of the medical genetic significance of the mutation so that, if positive for the mutation, they will know its lifetime significance and pertinent penetrance, and, importantly, will receive a full description of the surveillance recommendations. It is extremely important that the genetic counselor spend as much time as necessary assuring that the patient has fully understood the implications of a deleterious germline mutation so that morbidity and mortality may be significantly reduced. The other issue pertains to the individual who is found to be negative for the family’s deleterious mutation. This person must be told how common cancer is and that, while a “negative” result means that he/she will not be under the yoke of a highly significant life-long risk for syndrome cancer, nevertheless, he/she will still harbor the general population risk for these particular cancers.

Full table
Discussion
The fields of hereditary cancer and molecular genetics have advanced so rapidly that it is extremely difficult for physicians to keep up with this explosive knowledge. Clearly, the issue is “who is going to take care of all these crucial matters for patient benefit?” This is a germane question and our experience has confirmed that, in addition to certified genetic counselors, advanced practice oncology nurses who are interested in hereditary cancer can become skilled at providing this service to the patient and his/her family. This is, in fact, how our HCC has evolved, namely, an extremely well-informed oncology nurse with 20 years of experience in genetic counseling and hereditary cancer, along with availability of physician molecular genetics and pathology colleagues.
Physicians and genetic counselors rarely if ever conduct outreach activities to make contact with distant relatives in the clinical setting due to shortage of time, lack of compensation, and concerns about confidentiality and privacy. Indeed, notions of “duty to warn” are sufficiently vague, both in principle and in practice, as to deter even highly motivated clinicians (15). What inroads have been made have occurred in the setting of research studies. Such studies have shown that interventions to communicate risk information can be effectively conducted (16,17).
A key issue in LS is the lack of ascertainment of relatives of probands with germline mismatch repair (MMR) mutations. Attention is usually given to the proband’s first-degree relatives (children and siblings) in the sense that mutation-positive patients can appreciate the immediacy of the genetic risk to these individuals. Index cases can usually be counted on to provide siblings and adult children with a copy of the “family letter” that is commonly provided at the conclusion of a results disclosure genetic counseling session or to otherwise communicate the substance of the information that has been given. Multiple studies have reported that communication of genetic risk information to first-degree relatives is common; in fact, learning cancer risk information for one’s relatives is often a primary motivator to pursue genetic counseling and testing (17-19).
The U.S. Department of Health and Human Services, through its Healthy People 2020 initiative (20), has deemed education of relatives to be a priority, stating that “All people who are newly diagnosed with CRC should receive counseling and educational materials about genetic testing. Family members could benefit from knowing whether the CRC in their family is a hereditary form called LS (2). Screening interventions could potentially reduce the risk of CRC among men and women with LS by 60% (21).” The efficacy of CRC surveillance has been demonstrated by a number of studies, including a 15-year trial by Järvinen et al. (22) which showed that colonoscopic screening of LS family members reduced the risk of CRC, prevented CRC deaths through early detection, and decreased overall morbidity and mortality. de Jong et al. (23) among others (16,24) found benefit in colonoscopic surveillance in LS family members. Several studies (25-27) have found positive correlation between genetic counseling and uptake of CRC screening. A previous decision analysis (28) suggests that screening of LS patients “…can yield substantial benefits at acceptable costs, presuming sufficient uptake of genetic testing by first-degree relatives of LS probands”.
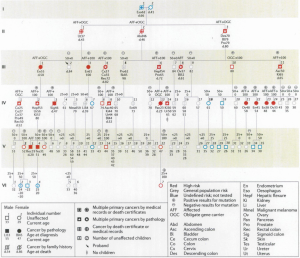
The benefits of genetic testing can extend beyond those tested, changing the known risk status of other family members. Watson et al. (29) investigated the change in the distribution of carrier risk status resulting from DNA testing among 75 HBOC syndrome and 47 LS cancer-prone families from our hereditary cancer registry. This involved 10,910 cohort members. Findings showed a change in carrier risk status in 2,906 individuals following testing of 1,408 family members. The most common type of risk change for these individuals was from at risk to noncarrier status, which involved 77% of the risk changes. In addition, 12% were changed from low risk to known carrier status. Therefore, 89% of risk status changes based on testing were from uncertainty to certainty, findings which became integral to cancer prevention recommendations and which impacted the involved family members. Furthermore, 60% of persons with a carrier risk status change were not themselves tested but, rather, their risk status changed because of a relative’s test result. In order to provide a model for clinical diagnosis, germline mutation testing, and the entire process from the physician/genetic counselor, patient standpoint, we present a large LS family which we have had the privilege to diagnose, test, counsel, and manage (Figure 1).
Current challenges
Reaching high cancer risk individuals who might benefit from DNA testing brings up one of the biggest unmet needs in the diagnosis and management of hereditary cancer-prone families, namely the common lack of identification and education of at-risk relatives of those found to harbor deleterious germline mutations. These may include numerous individuals who are not aware of their hereditary cancer risk status or possibly even of their membership in a family prone to hereditary cancer. One of the strengths of a dedicated HCC is experience in dealing with this problem and the ability to assist mutation carriers in reaching both first-degree relatives as well as those more distantly related.
Advances in “precision oncology” or “personalized medicine” have exploded in recent years. This field deals with the matching of mutations present in a tumor with some of the most effective chemotherapy or other treatment options. Despite its promise, several barriers limit widespread clinical adoption: (I) the need to collect and properly store tissue; (II) the lack of cost-effective companion diagnostic tests; (III) limited funding for bioinformatics infrastructure; (IV) issues related to patient accrual in clinical trials targeting highly selected subsets of patients; (V) industry barriers that block rational combination regimens; and (VI) the need to better understand mechanisms of drug resistance and how to monitor patients for the emergence of resistance (30). Centers with expertise in cancer genetics are in a position to aid in overcoming these barriers.
Acknowledgements
Funding: This work was supported by revenue from Nebraska cigarette taxes awarded to Creighton University by the Nebraska Department of Health and Human Services. Funding was also received from Liz’s Legacy Fund, through Kicks for a Cure. Dr. HT Lynch’s work is partially funded through the Charles F. and Mary C. Heider Chair in Cancer Research, which he holds at Creighton University.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the State of Nebraska or the Nebraska Department of Health and Human Services.
References
- Hampel H, Frankel WL, Martin E, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol 2008;26:5783-8. [PubMed]
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med 2009;11:35-41. [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [PubMed]
- Watson P, Lynch HT. Cancer risk in mismatch repair gene mutation carriers. Fam Cancer 2001;1:57-60. [PubMed]
- Kwon JS, Scott JL, Gilks CB, et al. Testing women with endometrial cancer to detect Lynch syndrome. J Clin Oncol 2011;29:2247-52. [PubMed]
- Watson P, Vasen HF, Mecklin JP, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer 2008;123:444-9. [PubMed]
- Barrow E, Robinson L, Alduaij W, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet 2009;75:141-9. [PubMed]
- Lynch HT, Snyder C, Casey MJ. Hereditary ovarian and breast cancer: what have we learned? Ann Oncol 2013;24 Suppl 8: viii83-viii95.
- Fearon ER. Genetic alterations underlying colorectal tumorigenesis. Cancer Surv 1992;12:119-36. [PubMed]
- Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 2006;296:1507-17. [PubMed]
- Kwon JS, Sun CC, Peterson SK, et al. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome. Cancer 2008;113:326-35. [PubMed]
- Schmeler KM, Lynch HT, Chen LM, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med 2006;354:261-9. [PubMed]
- Vadaparampil ST, McIntyre J, Quinn GP. Awareness, perceptions, and provider recommendation related to genetic testing for hereditary breast cancer risk among at-risk Hispanic women: similarities and variations by sub-ethnicity. J Genet Couns 2010;19:618-29. [PubMed]
- Offit K. Clinical Cancer Genetics: Risk Counseling and Management. New York: Wiley-Liss Inc., 1998.
- Offit K, Groeger E, Turner S, et al. The "duty to warn" a patient's family members about hereditary disease risks. JAMA 2004;292:1469-73. [PubMed]
- Aktan-Collan K, Haukkala A, Pylvänäinen K, et al. Direct contact in inviting high-risk members of hereditary colon cancer families to genetic counselling and DNA testing. J Med Genet 2007;44:732-8. [PubMed]
- Peterson SK, Watts BG, Koehly LM, et al. How families communicate about HNPCC genetic testing: findings from a qualitative study. Am J Med Genet C Semin Med Genet 2003;119C:78-86. [PubMed]
- Wiseman M, Dancyger C, Michie S. Communicating genetic risk information within families: a review. Fam Cancer 2010;9:691-703. [PubMed]
- Chivers Seymour K, Addington-Hall J, Lucassen AM, et al. What facilitates or impedes family communication following genetic testing for cancer risk? A systematic review and meta-synthesis of primary qualitative research. J Genet Couns 2010;19:330-42. [PubMed]
- U.S. Department of Health and Human Services. Healthy People 2020 Topics and Objectives: Genomics. Accessed April 16, 2013. Available online: .http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=15
- Stupart DA, Goldberg PA, Algar U, et al. Surveillance colonoscopy improves survival in a cohort of subjects with a single mismatch repair gene mutation. Colorectal Dis 2009;11:126-30. [PubMed]
- Järvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer. Gastroenterology 2000;118:829-34. [PubMed]
- de Jong AE, Hendriks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006;130:665-71. [PubMed]
- Engel C, Rahner N, Schulmann K, et al. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol 2010;8:174-82. [PubMed]
- Burton-Chase AM, Hovick SR, Peterson SK, et al. Changes in screening behaviors and attitudes toward screening from pre-test genetic counseling to post-disclosure in Lynch syndrome families. Clin Genet 2013;83:215-20. [PubMed]
- Armelao F, Orlandi PG, Tasini E, et al. High uptake of colonoscopy in first-degree relatives of patients with colorectal cancer in a healthcare region: a population-based, prospective study. Endoscopy 2010;42:15-21. [PubMed]
- Ueland AS, Hornung PA, Greenwald B. Colorectal cancer prevention and screening: a Health Belief Model-based research study to increase disease awareness. Gastroenterol Nurs 2006;29:357-63. [PubMed]
- Ladabaum U, Wang G, Terdiman J, et al. Strategies to identify the Lynch syndrome among patients with colorectal cancer: a cost-effectiveness analysis. Ann Intern Med 2011;155:69-79. [PubMed]
- Watson P, Narod SA, Fodde R, et al. Carrier risk status changes resulting from mutation testing in hereditary non-polyposis colorectal cancer and hereditary breast-ovarian cancer. J Med Genet 2003;40:591-6. [PubMed]
- Kaufman HL. Precision immunology: the promise of immunotherapy for the treatment of cancer. J Clin Oncol 2015;33:1315-7. [PubMed]


