Lynch syndrome: expanded tumor spectrum, universal screening and multimodal treatment strategies for colon cancer
Introduction
A total of 15% to 30% of colorectal cancers (CRC) are considered familial, defined as a positive family history of cancer in one or more first degree relatives (1). Lynch syndrome (LS) accounts for 2% to 6% of all cases of CRC (2-6). In 2014, there are 136,830 new cases of CRC diagnosed in the United States (7), therefore, as many as 8,210 of those cancers could be in patients with LS.
The majority of LS associated tumors demonstrate microsatellite instability (MSI), indicating a malfunction of the DNA mismatch repair (MMR) mechanism. In most LS kindreds, a germline mutation in one of four MMR genes (MLH1, MSH2, MSH6, or PMS2) has been detected. The genotypes MLH1 and MSH2 account for approximately 50% and 40% of the germline mutations, respectively. MSH6 represents approximately 10% of the germline mutations, while PMS2 accounts for a much smaller fraction of cases (8).
The MMR defects are inherited in autosomal dominant fashion. Thus, half of LS kindred members will be carriers of a mutation that predisposes them to early onset cancers. LS mutation carriers have been estimated to carry lifetime risks of colorectal and endometrial cancers as high as 82% and 60%, respectively. Increased risks of extracolonic and non-endometrial cancers in decreasing order of frequency are urinary tract (8.4%), ovaries (6.7%), stomach (5.8%, higher incidence in Asian countries such as Korea and Japan), small bowel (4.3%), hepatobiliary-pancreatic tumors (4.1%), and brain (2.1%) (9).
Expanded tumor spectrum
Recently, MSI has been identified in breast, bladder and prostate cancers in patients with LS (10,11). Bladder cancer is a recent addition to LS since 2010 (12-14). In a current study on LS tumor spectrum, we have shown an increased lifetime risk of urinary tract cancers (UTC), specifically urothelial cancers inclusive of renal pelvis, ureter and bladder, 13.4% in MSH2 germline mutation carriers versus 1.5% in MLH1 carriers (15). Exclusive bladder cancer risk (up to age 70) in MSH2 men is 7.5% vs. 1% for MSH2 women (12).
Per national SEERS data, the lifetime risk of both men and women developing bladder cancer at some point in their life is 2.4%, which in turn is estimated to be 4.5% (n=74,000) of all new cancer cases for 2014 (16). Thus due to the addition of bladder as a Lynch tumor, UTC is currently considered the 3rd most common malignancy in LS mutation carriers, particularly MSH2 males, henceforth surveillance strategies for urothelial cancers should be strongly considered in this subgroup. Other rare extracolonic cancers, such as sarcomas and adrenal carcinoma, have also been observed in certain LS families (17,18). As more DNA research of tumors is performed, the overall tumor spectrum in LS is predicted to expand.
Universal testing of all patients with CRC
Patients with potential LS may now be initially screened and identified by testing their cancer specimens for MSI and utilizing immunohistochemistry (IHC) (7,19). Family history can further identify these patients as suspected members of a LS family who could benefit from genetic testing. The Amsterdam II and revised Bethesda clinical criteria have been created to identify kindred members at risk for LS (20), especially those with strong family history for colorectal and endometrial cancers. However, recent studies have argued for universal testing of all CRC patients for MSI regardless of family history, and have even demonstrated cost-effectiveness (2,21,22).
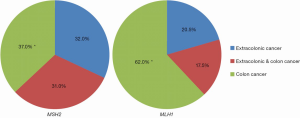
Currently no clinical criteria is highly effective in identifying LS patients presenting with extracolonic cancers only in the absence of CRC. By not including these patients perhaps for MSI testing of their cancer specimens, the effectiveness in identifying LS families is greatly diminished. Our recently published study shows that this is especially true in MSH2 kindreds who have an excess of extracolonic cancer only manifestation without CRC (32%) (Figure 1) (15). Thus, the application of MSI and MMR-IHC specimen testing in potential LS cancer patients based on family history is likely to expand.
Surgical strategy for LS colon cancer
Due to the increased cumulative risk for metachronous colon cancers versus the general population (2% per year vs. 0.3% per year) over the first 10 years post-op and beyond, a subtotal colectomy with ileorectal anastomosis (IRA) is the preferred surgical option for LS colon cancer, and for large premalignant polyps that cannot be managed with complete endoscopic removal of the polyps (23). This recommendation is based on data from several retrospective studies that have demonstrated when compared to subtotal colectomy, patients who undergo segmental resection of the colon are at higher risk for developing metachronous CRC (15.7-25% vs. 3.4-8%), as well as high-risk adenomas (22% vs. 11%) (24-26). Thus to reduce the risk of developing metachronous colon cancers, current data supports subtotal colectomy over segmental colectomy. Hemicolectomy may be considered over total or subtotal colectomy in elderly patients who are greater than 60-65 years old. The American College of Gastroenterology (ACG) guidelines cite that life expectancy gained based on Markov modeling was only 0.3 years in a 67 year-old undergoing a total colectomy versus a hemicolectomy. Therefore in an older patient, a hemicolectomy is a reasonable alternative.
In terms of mid to low rectal cancer, low anterior resection (LAR) or abdominal perineal resection (APR) is the standard procedure. Total proctocolectomy with ileal pouch anal anastomosis (IPAA) can be considered for younger patients (23). However, patients with low rectal cancer who undergo proctectomy only with coloanal anastomosis but without a colectomy will have a high cumulative risk of developing metachronous colon cancer at 10, 20, and 30 years following proctectomy with rates of 19%, 47%, and 69%, respectively. Thus, extensive colectomy will substantially reduce the risk of developing metachronous CRC to 0-3.4% (27).
Post-operative endoscopic surveillance
Because LS patients are at much higher risk for developing metachronous CRC compared to the sporadic population, strict post-operative colon surveillance is vitally important for continued management. Unfortunately, post-operative surveillance strategy following colectomy has not been well-studied. Current surveillance protocol dictates that LS patients who had subtotal colectomy undergo rigid proctoscopy or flexible sigmoidoscopy every 1-2 years. However, LS patients who had segmental colectomy also require colonoscopy every 1-2 years. This practice is supported by data found in a review of 110 LS patients which showed that nine patients developed a Dukes A, B, or C CRC within 2 years of negative history and physical examination findings (26), thus validating the results of a previous study which found that 8% of LS patients developed metachronous CRC within 5 years of colon resection (28).
Adjuvant chemotherapy for LS colon cancer
Since the late 1990’s the genetic abnormalities, microsatellite instability-high (MSI-H) or mismatch repair-deficient (MMRd), has shown prognostic importance in both node negative (Stage II) and node positive (Stage III) colon cancer. Patients with MSI-H/MMRd have a more favorable prognosis, stage for stage, when compared to microsatellite instability low (MSI-L), microsatellite stable (MSS) or mismatch repair proficient (MMRp) patients.
In 2003, Ribic has demonstrated the prognostic effects of MSI status on available colon cancer tissue samples from five randomized controlled trials (RCT) in patients undergoing adjuvant chemotherapy protocols, that were initiated in the late 1970’s to the late 1980’s (29). The majority of these chemotherapy trials are assessing 5-Fluorouracil (5-FU) plus/minus non-chemotherapeutic agent versus no chemotherapy. Of the 1,952 patient tumor samples randomized within these trials, 570 are analyzed for MSI and 95 patient samples (16.7%) are MSI-H. This study shows that MSI-H patients who did not receive any chemotherapy actually demonstrated remarkably better prognosis (HR for death 0.31) than those MSI-H patients who did receive chemotherapy, which affirmed the results of earlier nonrandomized retrospective studies. This improvement in prognosis also persists in subset analysis by stage of colon cancer. However, LS patients make up a minority of the MSI-H/MMRd colon cancer specimens since the majority of these are somatic mutations caused by the epigenetic inactivation/hypermethylation of the MLH1 gene.
On the contrary, in MSI-L/MSS patients, use of adjuvant chemotherapy does correlate with significant improvement in overall and disease free survival, when compared to MSI-L/MSS patients who did not receive chemotherapy. In fact, the prognoses of the combined MSI-L/MSS chemotherapy treated patients are no different from the prognosis shown by untreated patients with the MSI-H/MMRd genetic status. Accordingly, MSI-H chemotherapy patients actually display detrimental effect of chemotherapy as a group and also when subset analyzed for stage of colon cancer (Stage II HR for death 3.28 and for Stage III HR 1.42). These results have been reconfirmed by Sargent in 2010 (30), using tissue samples from different patients of the RCTs that Ribic previously drew from as well as from the Intergroup 0035 RCT. These studies have thus led to an assumption that 5-FU therapies are likely not beneficial, but could even be harmful when used in MSI-H/MMRd colon cancer patients.
Sinicrope in 2011 explores not only the effect of MMRd status on overall prognosis and the response to adjuvant chemotherapy but also attempts to differentiate results by categorizing patients as likely LS germline mutations versus likely somatic mutations (31). The patients are categorized as likely germline mutations if they showed loss of MSH2/MLH1 expression on the tumor specimen or are MSI-H and 55 years old or younger. None of these patients are BRAF gene mutated. Patients are categorized as sporadic, likely somatic mutations if they showed MSI-H on the tumor specimen or loss of MSH2/MLH1 and are over age 55. To clarify, many of the tumor samples are from the same trials quoted in the previous studies but this study also includes trials involving immunotherapy, portal vein infusion therapy, and multiagent chemotherapy.
In contradistinction to Ribic and Sargent’s findings, patients with MMRd, when treated with adjuvant 5-FU based regimens, show a benefit for Stage III colon cancer when compared with MMRd patients not treated. More importantly, when patients are categorized as ‘likely germline mutations’ (LS) and ‘likely somatic mutations’ and then compared in subset analysis, it appears that only patients with likely germline mutations (LS) benefited from adjuvant 5-FU therapy. Overall, this study shows that 5-FU based regimens could be beneficial in a specific subset of patients with MSI-H/MMRd tumor specimens if they have corresponding germline mutations (LS).
Sinicrope and colleagues as well as all other studies on this subject, are limited by their retrospective analysis, because patients are not prospectively randomized to chemotherapy versus no chemotherapy based on their MMR/MSI status. Furthermore, these papers involve small to moderate numbers of patients, which greatly affect whether or not this data shall be considered applicable at all to clinical practice involving LS patients. Additionally, no long-term outcomes from RCT of oxaliplatin (OX) or irinotecan based adjuvant chemotherapy have been analyzed to date for prognosis based on MSI-H/MMRd status. Furthermore, the majority of sporadic colon cancer patients are not MSI-H/MMRd. Considering these issues, it will be prudent currently to ignore the MSI-H/MMRd and LS status when considering adjuvant OX based chemotherapy, until enough non-5-FU based chemotherapy data on MSI-H tumors is collected to form a more concrete evidence based conclusion.
Lastly, the National Comprehensive Cancer Network (NCCN) colon cancer guidelines published on October 2014 (32) base their viewpoints primarily on the work of Ribic and Sargent, suggesting that MSI-H/MMRd and LS status be considered when assessing a patient for 5-FU based adjuvant chemotherapy alone. It further recommends that adjuvant 5-FU therapy be avoided in MSI-H/MMRd patients with Stage II colon cancer. However, the authors of this paper recommend that the literature data should be reevaluated and that Sinicrope’s study, which showed that 5-FU adjuvant therapy is effective under a subset population analysis for MSI-H tumors, should also be taken into serious consideration when counseling LS patients with resected stage III colon cancer for adjuvant chemotherapy.
In summary, new research on LS has revealed a larger spectrum of cancers inclusive of prostate, breast, and even bladder cancers. Due to the inheritability of this dominant gene, testing for LS is highly recommended in patients with significant family history of colorectal and endometrial cancers; however broader clinical screening techniques are needed to account for the expanded tumor spectrum in LS to better identify patients at risk in order to implement early cancer screening, such as UTC screening for MSH2 carriers. Currently, universal testing of all colon cancer patients for MSI/MMR tumor status is encouraged, as a precursor to genetic testing for LS, which would for example lead to selection of individuals most likely to benefit from 5-FU based adjuvant chemotherapy especially for Stage III colon cancer. Clinical data also reveals that more extensive colectomy reduces the risk of developing metachronous colon cancers in non-elderly patients with LS. Thus, LS patients even after colorectal resections, require strict surveillance endoscopy every 1-2 years to detect for metachronous CRC. Finally, with increased clinician awareness and improved identification of LS patients, the surgical and adjuvant treatment options for their cancers such as colon cancer can be optimized and tailored for their survival benefit based on research data.
Acknowledgements
The authors would like to acknowledge Dr. Henry Lynch for his significant work in Lynch syndrome and the establishment of the Creighton University Hereditary Cancer Center and Registry.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: The views expressed in this manuscript are those of the authors alone, and do not reflect the opinions of the United States Government, Department of Defense or the United States Army.
References
- van Wezel T, Middeldorp A, Wijnen JT, et al. A review of the genetic background and tumour profiling in familial colorectal cancer. Mutagenesis 2012;27:239-45. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Bonis PA, Trikalinos TA, Chung M, et al. Hereditary nonpolyposis colorectal cancer: diagnostic strategies and their implications. Evid Rep Technol Assess (Full Rep) 2007.1-180. [PubMed]
- Hampel H, Frankel WL, Martin E, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med 2005;352:1851-60. [PubMed]
- Samowitz WS, Curtin K, Lin HH, et al. The colon cancer burden of genetically defined hereditary nonpolyposis colon cancer. Gastroenterology 2001;121:830-8. [PubMed]
- Barnetson RA, Tenesa A, Farrington SM, et al. Identification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancer. N Engl J Med 2006;354:2751-63. [PubMed]
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [PubMed]
- Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med 2003;348:919-32. [PubMed]
- Chung DC, Rustgi AK. The hereditary nonpolyposis colorectal cancer syndrome: genetics and clinical implications. Ann Intern Med 2003;138:560-70. [PubMed]
- Buerki N, Gautier L, Kovac M, et al. Evidence for breast cancer as an integral part of Lynch syndrome. Genes Chromosomes Cancer 2012;51:83-91. [PubMed]
- Bauer CM, Ray AM, Halstead-Nussloch BA, et al. Hereditary prostate cancer as a feature of Lynch syndrome. Fam Cancer 2011;10:37-42. [PubMed]
- van der Post RS, Kiemeney LA, Ligtenberg MJ, et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J Med Genet 2010;47:464-70. [PubMed]
- Skeldon SC, Semotiuk K, Aronson M, et al. Patients with Lynch syndrome mismatch repair gene mutations are at higher risk for not only upper tract urothelial cancer but also bladder cancer. Eur Urol 2013;63:379-85. [PubMed]
- Bernstein IT, Myrhøj T. Surveillance for urinary tract cancer in Lynch syndrome. Fam Cancer 2013;12:279-84. [PubMed]
- Lin-Hurtubise KM, Yheulon CG, Gagliano RA Jr, et al. Excess of extracolonic non-endometrial multiple primary cancers in MSH2 germline mutation carriers over MLH1. J Surg Oncol 2013;108:433-7. [PubMed]
- Howlader N, Noone AM, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975-2012. Bethesda: National Cancer Institute, 2015. Available online: http://seer.cancer.gov/csr/1975_2012/
- Watson P, Riley B. The tumor spectrum in the Lynch syndrome. Fam Cancer 2005;4:245-8. [PubMed]
- Medina-Arana V, Delgado L, Bravo A, et al. Tumor spectrum in lynch syndrome, DNA mismatch repair system and endogenous carcinogens. J Surg Oncol 2012;106:10-6. [PubMed]
- Peltomäki P, Gao X, Mecklin JP. Genotype and phenotype in hereditary nonpolyposis colon cancer: a study of families with different vs. shared predisposing mutations. Fam Cancer 2001;1:9-15. [PubMed]
- Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261-8. [PubMed]
- Blokhuis MM, Pietersen GE, Goldberg PA, et al. Lynch syndrome: the influence of environmental factors on extracolonic cancer risk in hMLH1 c.C1528T mutation carriers and their mutation-negative sisters. Fam Cancer 2010;9:357-63. [PubMed]
- Shia J. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part I. The utility of immunohistochemistry. J Mol Diagn 2008;10:293-300. [PubMed]
- Syngal S, Brand RE, Church JM, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015;110:223-62. [PubMed]
- Kalady MF, McGannon E, Vogel JD, et al. Risk of colorectal adenoma and carcinoma after colectomy for colorectal cancer in patients meeting Amsterdam criteria. Ann Surg 2010;252:507-11; discussion 511-3. [PubMed]
- Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011;60:950-7. [PubMed]
- de Vos tot Nederveen Cappel WH, Nagengast FM, Griffioen G, et al. Surveillance for hereditary nonpolyposis colorectal cancer: a long-term study on 114 families. Dis Colon Rectum 2002;45:1588-94.
- Win AK, Parry S, Parry B, et al. Risk of metachronous colon cancer following surgery for rectal cancer in mismatch repair gene mutation carriers. Ann Surg Oncol 2013;20:1829-36. [PubMed]
- Lanspa SJ, Jenkins JX, Cavalieri RJ, et al. Surveillance in Lynch syndrome: how aggressive? Am J Gastroenterol 1994;89:1978-80. [PubMed]
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247-57. [PubMed]
- Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219-26. [PubMed]
- Sinicrope FA, Foster NR, Thibodeau SN, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst 2011;103:863-75. [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), Colon Cancer. Version 3. 2015. Available online: http://www.nccn.org