An update on the management of intrahepatic cholangiocarcinoma
Introduction
Together with peri-hilar cholangiocarcinoma and cholangiocarcinoma of distal common bile duct, intrahepatic cholangiocarcinoma (ICC) is a subtype of the cholangiocarcinoma family, contributing to 20–25% all cholangiocarcinoma cases (1). Despite this rarity, it is the second most common primary liver cancer following hepatocellular carcinoma (HCC), accounting to 10% of all primary liver cancer (2). Unlike the more common extrahepatic cholangiocarcinoma, a rising incidence of ICC was observed worldwide. In United States, the incidence had increased from 0.32 per 100,000 in 1975 to 0.85 per 100,000 in 1995 (3,4). Similar rise had been reported in both Far East and the West, such as Japan and United Kingdom (5,6). While some studies attributed it to different classification systems used by the epidemiological analysis (7), this rising trend has been stabilized in the last decade (8). Disease conditions like recurrent pyogenic cholangitis (RPC), choledochal cyst, biliary cirrhosis, primary sclerosing cholangitis (PSC), biliary papillomatosis/adenoma, alcoholic liver disease and parasitic infestations are known predisposing factors for development of ICC (9). Although diabetes mellitus, hepatitis B/C infection, nonalcoholic steatohepatitis, chronic pancreatitis, obesity and smoking are suggested to be associated with ICC (10), many ICCs are developed de novo without any of the above factors (11,12). Histologically, ICC can be classified into three distinct subtypes: mass-forming, peri-ductal infiltrating and intra-ductal growth (13). Mass-forming ICCs are the most common subtypes and have the propensity of intrahepatic metastasis; peri-ductal infiltrating confers the worst prognosis with its tendency to disseminate via hilar lymphatics.
Investigation and diagnosis
Since most of the ICC developed at the periphery of the biliary tree, obstructive jaundice is uncommon and most patients present late due to its insidious onset (14), around 12–30% of the ICC were diagnosed in asymptomatic patients (12,15) and curative surgery is possible only in about 40% of the patients (16). Cancer antigen 19–9 elevation might be found in some of the patient but it is not diagnostic (17). Tumour biopsy with the use of immunohistochemical tests such as a positive cytokeratin (CK) 7, negative CK20, negative transcription termination factor RNA polymerase (TTF1), negative deletion in pancreatic cancer, locus 4 (DPC4) and negative caudal type homeobox2 (CDX2) are evidence support the diagnosis of primary ICC (18). Tumour biopsy is usually reserved for inoperable disease as the sampling process might risk needle tract and peritoneal seedling.
Cross-sectional imaging remains the most important investigation modality for ICC. The proportion of tumour cells and fibrous tissue possessed by the tumour would affect its enhancement pattern and appearance on CT scan (19).
The typical CT features of a mass-forming cholangiocarcinoma include homogeneous attenuation, irregular peripheral enhancement with gradual centripetal enhancement, capsular retraction, the presence of satellite nodules, and associated intra-hepatic ductal dilatation around the tumour (13,20-23). If ICC developed in background RPC, there may be co-existing hepaticolithiasis, dilated intrahepatic duct, atopic hepatic parenchyma and thrombosed portal vein to the diseased lobe (24). For peri-ductal infiltrative ICC, it appears as enhancing peri-ductal thickening around a dilated or narrowed intrahepatic bile duct without mass formation resembling that of infiltrative hilar cholangiocarcinoma (23). In case of intra-ductal type, it can have five imaging patterns, namely diffused and marked ductal ectasia with or without visible mass, intra-ductal polypoid mass within a dilated intra-hepatic duct, intra-ductal cast within a dilated duct and focal stricture associated with proximal ductal dilatation (25). However, due to presence of surrounding fibrous tissue, there is limitation for CT to delineate the extent of carcinoma and resectability can be determined in only 60% of the cases (26); another shortcoming of CT scan is it may not be able to differentiate PSC and ICC (27). ICCs are typically hypointense on T1 weighted and hyperintense on T2 weighted imaging in MRI. Peripheral enhancement and progressive concentric filling would be seen in delay phases of contrast MRI (28). However, some of the ICC exhibit atypical imaging features and by the same token, there are a number of mimickers for ICC. In cirrhotic patients, HCC with cirrhotic stroma, sclerosing HCC and cholangiohepatocellular carcinoma could have nearly identical imaging characteristics as ICC (29-31). Recent studies suggest that, use of a new liver specific MRI contrast, Gadolinium ethoxybenzyl dimeglumine, also known as gadoxetate disodium, can increase the conspicuity and better delineate characteristics of various liver tumours (32,33).
Fluorodeoxyglucose (FDG)-PET has a specific role in the management of ICC, some studies reported a high sensitivity and specificity of 85% for diagnosis of ICC (34,35), and small cholangiocarcinoma of 1 cm can also be detected, despite this is less sensitive in presence of inflammatory process or infiltrative type ICC (34,36,37). In addition, FDG-PET is more specific in picking up regional lymphadenopathy than CT scan, though it is not more sensitive (35,38).. While at most of the time, FDG-PET reveals a “hot” spot in the liver and do not differentiate ICC from other tumours, some investigators reported that FDG-PET can change the management of up to 30% of the patients with ICCs by detecting metastatic diseases (35,39,40). Therefore, FDG-PET should be considered in patients with systemic symptoms or locally advanced tumour.
Staging for intrahepatic cholangiocarcinoma (ICC)
Staging system plays an important role in prognosticating and guiding treatment of ICC. In 1997, The Liver Cancer Study Group of Japan (LCSGJ) first advocated an independent staging for ICC from HCC (41). There were two Japanese systems proposed by Yamasaki (13) and Okabayashi (42) respectively. The former one staged ICC by solitary tumour size cut-off at 2 cm, venous invasion and presence peritoneal metastasis while the other one employed presence of symptoms, lymph node metastasis, multifocal tumour, vascular invasion and peritoneal metastasis for staging. Outside Japan, AJCC/UICC classification is commonly used. Like the classification in Japan, size of tumour remains an important factor to assign the T-staging in sixth edition AJCC/UICC (43). However, since this system was derived from data largely based on HCC patients, and due to the differences in carcinogenesis mechanism and tumor biology between HCC and ICC (44-48), AJCC/UICC sixth edition was criticized to be lacking of discriminatory ability. Subsequently, a large SEER cohort performed in 2009 (49) demonstrated that tumour size is not associated with long-term survivals. The simplified staging system proposed was noted to have better discriminatory ability than AJCC/UICC sixth edition and superior discriminatory power when compared to Japanese staging systems (49). Based on these findings, the T-staging criteria in the latest AJCC/UICC seventh edition (50) exclude tumour size. In addition, a number of studies (45,49-57) validated the prognostic significance of vascular invasion and multiple tumour; with the latter being regarded as a worse parameter by some authors (52,53). Apart from the newly added criteria of peri-ductal infiltration as T4 disease, tumour involvement of regional lymph node is currently regarded as stage IVa disease instead of stage III disease as in the sixth edition. This is because the impact of lymph node metastasis on survival is even stronger than that of the vascular invasion and multifocal disease (58). Some studies suggest the number of lymph node metastasis (49,54,59,60) and the ratio of positive lymph node to examined lymph node (54) have implication on prognosis. However, there is no sub-classification of the extent of lymph node metastasis in the AJCC seventh edition, albeit, this staging system is externally and independently validated by the French Surgical Association (AFC)-IHCC 2009 study group and hence recommended for both clinical and research purposes (61).
Surgical treatments of intrahepatic cholangiocarcinoma (ICC)
Resection remains the only hope of cure in patients with ICC (62-64), but for a multitude of reasons, R0 resection (macroscopic and microscopic negative margin) is only achieved in 32% to 96% of the cases (15,52,55,58,60-62,65-69). Intrahepatic metastasis and peritoneal deposits are some of the reasons for unresectability, some studies suggested that diagnostic laparoscopy can save unnecessary laparotomy from 27–36% of the cases (66,70). However, other studies reported that the sensitivity of staging laparoscopy in only 55% (66) and a high false negative rate (70). Furthermore, many of the unresectable cases are due to major vascular invasion or contralateral biliary system involvement that could only be confirmed with surgical dissection. Therefore, staging laparoscopy could not be recommended as a routine until further convincing data emerged.
Resection of ICC needs to be aggressive in order to obtain the best oncological outcomes (65,71,72). It has been suggested that, for mass forming type of ICC, operation should follow the principle of anatomical major hepatectomy, while for infiltrative types ICC, extrahepatic bile duct excision and lymph node dissection should be performed (73,74). Extended hepatectomy, bile duct reconstruction and vascular resection are frequently required, reported as up to 78%, 29% and 14% respectively (15,55,60,66,67,69,75,76), despite the complexity of the surgery, the reported morbidity and mortality in some centers can be as low as 6% and 1% (10,49,55) respectively. Therefore, hepatectomy for ICC is best to be performed in high volume center (77).
Advances of surgical technique and perioperative care allow many challenging ICC to become resectable, however, the prognosis for this group of patients remains poor with 5-year overall survival (OS) less than 40% in most recent series (Table 1). Regional lymphadenectomy and wider resection have been the area of active research in the hope that the survival could be further prolonged. Routine lymphadenectomy could theoretically enhance oncological clearance (74,80) and optimize pathological staging, however, the counter-argument is lymph node dissection does not seem to influence ultimate survival of ICC patients (57,81,82). While strong evidence is lacking, lymph node dissection is sensible when there is gross regional lymph node involvement. Concerning the resection margin issue, there is little controversy about the benefit of clear resection margin (52,60,62,66,83,84), the definition of adequate margin width remains a topic of debate (85-88). We had recently reviewed our patients who has resection for ICC and found that margin width tends to be more important in patients who have solitary and node negative tumour, this finding is echoed the another study by Farges et al. (84).
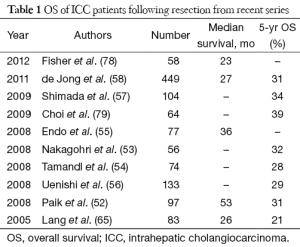
Full table
Liver transplantation is a well-recognized treatment for HCC with 5-year survival ranges from 71% to 87% in patient within certain criteria (89-93). However, similar result was not reproduced and poor outcomes were reported in patients with ICC receiving liver transplantation (94,95). A large cohort for liver transplantation for ICC extracted from the European Liver Transplant showed that the 5-year survival was only 29% (96), other studies suggest high tumour recurrence rate of 51% to 80% (97,98). Liver transplantation for ICC should be limited to highly selected patients or under research setting.
Non-surgical treatment for intrahepatic cholangiocarcinoma (ICC)
Transarterial chemoembolization (TACE) and transarterial radioembolization (TARE) are established treatments for unresectable HCC without extrahepatic disease. Despite these treatments were intrinsically designed for hypervascular tumours like HCC, it has been applied to ICC. A number of case series has suggested TACE/TARE possess satisfactory safety profile and efficacy with response rate of 70–86% and median survival ranging from 11 to 13 months (99-101).
External radiotherapy could be given to patients with unresectable ICC hoping to provide local control and prolong survival. A 36% response rate was reported for patients who receive a median total of 50 Gy given in 2 Gy per section 5 times a week (102). In order to minimize radiation exposure to the normal liver tissue, stereotactic radiotherapy technique can be used (103). A study reported that, with the use of lower radiation dose 30 Gy, the disease control and median survival after receiving stereotactic radiotherapy was 55% and 11 months respectively with significant toxicity was observed (104). Adjuvant radiotherapy after curative surgery was shown by a retrospective series to be better than surgery alone (105). However, before the emergence of higher level of evidence or further clinical studies, adjuvant radiotherapy should be recommended in selected patients, i.e., positive margin, or nodal disease (106).
Being a mediator of hepatic inflammatory process, cholangiocyte is equipped with the ability to initiate and maintain carcinogenesis, making cholangiocarcinoma inherently resistant to chemotherapy (107,108). Response rate for classical single agent gemcitabine is at best 30% with median survival of 5–14 months (109-114). As proven by some clinical trials, combining gemcitabine with platinum group chemotherapeutic agent can increase response rate to up to 50%, and median survival of the patients was also prolonged (115-117), such that combination gemcitabine and oxaliplatin/cisplatin has become the standard chemotherapeutic regime in many centers. Furthermore, advances in bioinformatics would allow more oncogenes taking part in the carcinogenetic pathway for ICC to be identified (118); further clinical trials focusing on combination chemotherapy with targeted therapy are expected to be emerging (119-121).
Conclusions
ICC is an uncommon and sinister hepatic malignancy. Predisposed patient groups should be offered surveillance with sophisticated imaging modalities. Diagnosis at early stage with aggressive surgical resection remains the only hope of cure in a small proportion of the ICC patients. Various non-surgical treatments are available but the prognoses are generally guarded. Further clinical trials focusing on newly chemotherapeutic and molecular targeted regimen are early awaited.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Khan SA, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut 2002;51 Suppl 6:VI1-9. [PubMed]
- Aljiffry M, Abdulelah A, Walsh M, et al. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg 2009;208:134-47. [PubMed]
- Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002;2:10. [PubMed]
- Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. [PubMed]
- Kato I, Kuroishi T, Tominaga S. Descriptive epidemiology of subsites of cancers of the liver, biliary tract and pancreas in Japan. Jpn J Clin Oncol 1990;20:232-7. [PubMed]
- Taylor-Robinson SD, Foster GR, Arora S, et al. Increase in primary liver cancer in the UK, 1979-94. Lancet 1997;350:1142-3. [PubMed]
- Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol 2012;56:848-54. [PubMed]
- Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology 2009;136:1134-44. [PubMed]
- Sorensen HT, Friis S, Olsen JH, et al. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology 1998;28:921-5. [PubMed]
- Brown KM, Parmar AD, Geller DA. Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am 2014;23:231-46. [PubMed]
- Sempoux C, Jibara G, Ward SC, et al. Intrahepatic cholangiocarcinoma: new insights in pathology. Semin Liver Dis 2011;31:49-60. [PubMed]
- Dhanasekaran R, Hemming AW, Zendejas I, et al. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol Rep 2013;29:1259-67. [PubMed]
- Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg 2003;10:288-91. [PubMed]
- de Groen PC, Gores GJ, LaRusso NF, et al. Biliary tract cancers. N Engl J Med 1999;341:1368-78. [PubMed]
- Shen WF, Zhong W, Xu F, et al. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma. World J Gastroenterol 2009;15:5976-82. [PubMed]
- Tan JC, Coburn NG, Baxter NN, et al. Surgical management of intrahepatic cholangiocarcinoma--a population-based study. Ann Surg Oncol 2008;15:600-8. [PubMed]
- Patel AH, Harnois DM, Klee GG, et al. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol 2000;95:204-7. [PubMed]
- Maithel SK, Gamblin TC, Kamel I, et al. Multidisciplinary approaches to intrahepatic cholangiocarcinoma. Cancer 2013;119:3929-42. [PubMed]
- Miura F, Okazumi S, Takayama W, et al. Hemodynamics of intrahepatic cholangiocarcinoma: evaluation with single-level dynamic CT during hepatic arteriography. Abdom Imaging 2004;29:467-71. [PubMed]
- Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR Am J Roentgenol 2003;181:819-27. [PubMed]
- Ros PR, Buck JL, Goodman ZD, et al. Intrahepatic cholangiocarcinoma: radiologicpathologic correlation. Radiology 1988;167:689-93. [PubMed]
- Choi BI, Lee JH, Han MC, et al. Hilar cholangiocarcinoma: comparative study with sonography and CT. Radiology 1989;172:689-92. [PubMed]
- Han JK, Choi BI, Kim AY, et al. Cholangiocarcinoma: pictorial essay of CT and cholangiographic findings. Radiographics 2002;22:173-87. [PubMed]
- Vilgrain V, Van Beers BE, Flejou JF, et al. Intrahepatic cholangiocarcinoma: MRI and pathologic correlation in 14 patients. J Comput Assist Tomogr 1997;21:59-65. [PubMed]
- Chung YE, Kim MJ, Park YN, et al. Varying appearances of cholan- giocarcinoma: radiologic-pathologic correlation. Radiographics 2009;29:683-700. [PubMed]
- Zhang Y, Uchida M, Abe T, et al. Intrahepatic peripheral cholangiocarcinoma: comparison of dynamic CT and dynamic MRI. J Comput Assist Tomogr 1999;23:670-7. [PubMed]
- Bhuiya MR, Nimura Y, Kamiya J, et al. Clinicopathologic studies on perineural invasion of bile duct carcinoma. Ann Surg 1992;215:344-9. [PubMed]
- Miller G, Schwartz LH, D’Angelica M. The use of imaging in the diagnosis and staging of hepatobiliary malignancies. Surg Oncol Clin N Am 2007;16:343-68. [PubMed]
- Lee WJ, Lim HK, Jang KM, et al. Radiologic spectrum of cholangiocarcinoma: emphasis on unusual manifestations and differential diagnoses. Radiographics 2001;21:S97-S116. [PubMed]
- Zuo HQ, Yan LN, Zeng Y, et al. Clinicopathological characteristics of 15 patients with combined hepatocellular carcinoma and cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 2007;6:161-5. [PubMed]
- Nishie A, Yoshimitsu K, Asayama Y, et al. Detection of combined hepatocellular and cholangiocarcinomas on enhanced CT: comparison with histologic findings. AJR Am J Roentgenol 2005;184:1157-62. [PubMed]
- Kang Y, Lee JM, Kim SH, et al. Intrahepatic mass-forming cholangiocarcinoma: enhancement patterns on gadoxetic acid-enhanced MR images. Radiology 2012;264:751-60. [PubMed]
- Péporté AR, Sommer WH, Nikolaou K, et al. Imaging features of intrahepatic cholangiocarcinoma in Gd-EOB-DTPA-enhanced MRI. Eur J Radiol 2013;82:e101-6. [PubMed]
- Anderson CD, Rice MH, Pinson CW, et al. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg 2004;8:90-7. [PubMed]
- Kim YJ, Yun M, Lee WJ, et al. Usefulness of 18F-FDG PET in intrahepatic cholangiocarcinoma. Eur J Nucl Med Mol Imaging 2003;30:1467-72. [PubMed]
- Fevery J, Buchel O, Nevens F, et al. Positron emission tomography is not a reliable method for the early diagnosis of cholangiocarcinoma in patients with primary sclerosing cholangitis. J Hepatol 2005;43:358-60. [PubMed]
- Fritscher-Ravens A, Bohuslavizki KH, Broering DC, et al. FDG PET in the diagnosis of hilar cholangiocarcinoma. Nucl Med Commun 2001;22:1277-85. [PubMed]
- Grobmyer SR, Wang L, Gonen M, et al. Perihepatic lymph node assessment in patients undergoing partial hepatectomy for malignancy. Ann Surg 2006;244:260-4. [PubMed]
- Corvera CU, Blumgart LH, Akhurst T, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg 2008;206:57-65. [PubMed]
- Petrowsky H, Wildbrett P, Husarik DB, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. J Hepatol 2006;45:43-50. [PubMed]
- The Liver Cancer Study Group of Japan. General Rules for the Clinical and Pathological Study of Primary Liver Cancer. First English edition. Tokyo: Kanehara & Company. Ltd., 1997.
- Okabayashi T, Yamamoto J, Kosuge T, et al. A new staging system for mass- forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer 2001;92:2374-83. [PubMed]
- Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed. New York: Springer-Verlag, 2002.
- Ikeda K, Saitoh S, Koida I, et al. A multivariate analysis of risk factors for hepatocellular carcinogenesis: a prospective observation of 795 patients with viral and alcoholic cirrhosis. Hepatology 1993;18:47-53. [PubMed]
- Inoue K, Makuuchi M, Takayama T, et al. Long-term survival and prognostic factors in the surgical treatment of mass-forming type cholangiocarcinoma. Surgery 2000;127:498-505. [PubMed]
- Parkin DM, Srivatanakul P, Khlat M, et al. Liver cancer in Thailand. I. A case-control study of cholangiocarcinoma. Int J Cancer 1991;48:323-8. [PubMed]
- Srivatanakul P, Parkin DM, Jiang YZ, et al. The role of infection by Opisthorchis viverrini, hepatitis B virus, and aflatoxin exposure in the etiology of liver cancer in Thailand. A correlation study. Cancer 1991;68:2411-7. [PubMed]
- Srivatanakul P, Parkin DM, Khlat M, et al. Liver cancer in Thailand. II. A case-control study of hepatocellular carcinoma. Int J Cancer 1991;48:329-32. [PubMed]
- Nathan H, Aloia TA, Vauthey JN, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol 2009;16:14-22. [PubMed]
- Edge S, Byrd DR, Compton CC, et al, editors. AJCC Cancer Staging Manual. 7th ed. New York: Springer-Verlag, 2010.
- Ohtsuka M, Ito H, Kimura F, et al. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg 2002;89:1525-31. [PubMed]
- Paik KY, Jung JC, Heo JS, et al. What prognostic factors are important for resected intrahepatic cholangiocarcinoma? J Gastroenterol Hepatol 2008;23:766-70. [PubMed]
- Nakagohri T, Kinoshita T, Konishi M, et al. Surgical outcome and prognostic factors in intrahepatic cholangiocarcinoma. World J Surg 2008;32:2675-80. [PubMed]
- Tamandl D, Herberger B, Gruenberger B, et al. Influence of hepatic resection margin on recurrence and survival in intrahepatic cholangiocarcinoma. Ann Surg Oncol 2008;15:2787-94. [PubMed]
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84-96. [PubMed]
- Uenishi T, Kubo S, Yamazaki O, et al. Indications for surgical treatment of intrahepatic cholangiocarcinoma with lymph node metastases. J Hepatobiliary Pancreat Surg 2008;15:417-22. [PubMed]
- Shimada K, Sano T, Nara S, et al. Therapeutic value of lymph node dissection during hepatectomy in patients with intrahepatic cholangiocellular carcinoma with negative lymph node involvement. Surgery 2009;145:411-6. [PubMed]
- de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5. [PubMed]
- Suzuki S, Sakaguchi T, Yokoi Y, et al. Clinicopathological prognostic factors and impact of surgical treatment of mass-forming intrahepatic cholangiocarcinoma. World J Surg 2002;26:687-93. [PubMed]
- Nakagawa T, Kamiyama T, Kurauchi N, et al. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg 2005;29:728-33. [PubMed]
- Farges O, Fuks D, Le Treut YP, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: By the AFC-IHCC-2009 study group. Cancer 2011;117:2170-7.
- DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007;245:755-62. [PubMed]
- Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996;224:463-73; discussion 473-5. [PubMed]
- Morise Z, Sugioka A, Tokoro T, et al. Surgery and chemotherapy for intrahepatic cholangiocarcinoma. World J Hepatol 2010;2:58-64. [PubMed]
- Lang H, Sotiropoulos GC, Frühauf NR, et al. Extended hepatectomy for intrahepatic cholangiocellular carcinoma (ICC): when is it worthwhile? Single center experience with 27 resections in 50 patients over a 5-year period. Ann Surg 2005;241:134-43. [PubMed]
- Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg 2001;193:384-91. [PubMed]
- Ali SM, Clark CJ, Zaydfudim VM, et al. Role of major vascular resection in patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol 2013;20:2023-8. [PubMed]
- Guglielmi A, Ruzzenente A, Campagnaro T, et al. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg 2009;33:1247-54. [PubMed]
- Konstadoulakis MM, Roayaie S, Gomatos IP, et al. Fifteen-year, single-center experience with the surgical management of intrahepatic cholangiocarcinoma: operative results and long-term outcome. Surgery 2008;143:366-74. [PubMed]
- Goere D, Wagholikar GD, Pessaux P, et al. Utility of staging laparoscopy in subsets of biliary cancers: laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma. Surg Endosc 2006;20:721-5. [PubMed]
- Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet 2005;366:1303-14. [PubMed]
- Neuhaus P, Jonas S, Settmacher U, et al. Surgical management of proximal bile duct cancer: extended right lobe resection increases resectability and radicality. Langenbecks Arch Surg 2003;388:194-200. [PubMed]
- Ohashi K, Nakajima Y, Tsutsumi M, et al. Clinical characteristics and proliferating activity of intrahepatic cholangiocarcinoma. J Gastroenterol Hepatol 1994;9:442-6. [PubMed]
- Yamamoto J, Kosuge T, Takayama T, et al. Surgical treatment of intrahepatic cholangiocarcinoma: four patients surviving more than five years. Surgery 1992;111:617-22. [PubMed]
- Sotiropoulos GC, Bockhorn M, Sgourakis G, et al. R0 liver resections for primary malignant liver tumors in the noncirrhotic liver: a diagnosis-related analysis. Dig Dis Sci 2009;54:887-94. [PubMed]
- Madariaga JR, Iwatsuki S, Todo S, et al. Liver resection for hilar and peripheral cholangiocarcinomas: a study of 62 cases. Ann Surg 1998;227:70-9. [PubMed]
- Nathan H, Cameron JL, Choti MA, et al. The volume-outcomes effect in hepato-pancreato-biliary surgery: hospital versus surgeon contributions and specificity of the relationship. J Am Coll Surg 2009;208:528-38. [PubMed]
- Fisher SB, Patel SH, Kooby DA, et al. Lymphovascular and perineural invasion as selection criteria for adjuvant therapy in intrahepatic cholangiocarcinoma: a multi-institution analysis. HPB (Oxford) 2012;14:514-22. [PubMed]
- Choi SB, Kim KS, Choi JY, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol 2009;16:3048-56. [PubMed]
- Shirabe K, Shimada M, Harimoto N, et al. Intrahepatic cholangiocarcinoma: its mode of spreading and therapeutic modalities. Surgery 2002;131:S159-64. [PubMed]
- Chu KM, Fan ST. Intrahepatic cholangiocarcinoma in Hong Kong. J Hepatobiliary Pancreat Surg 1999;6:149-53. [PubMed]
- Morine Y, Shimada M, Utsunomiya T, et al. Clinical impact of lymph node dissection in surgery for peripheral-type intrahepatic cholangiocarcinoma. Surg Today 2012;42:147-51. [PubMed]
- Cherqui D, Tantawi B, Alon R, et al. Intrahepatic cholangiocarcinoma. Results of aggressive surgical management. Arch Surg 1995;130:1073-8. [PubMed]
- Farges O, Fuks D, Boleslawski E, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg 2011;254:824-29; discussion 830. [PubMed]
- Shimada K, Sano T, Sakamoto Y, et al. Clinical impact of the surgical margin status in hepatectomy for solitary mass-forming type intrahepatic cholangiocarcinoma without lymph node metastases. J Surg Oncol 2007;96:160-5. [PubMed]
- Murakami S, Ajiki T, Okazaki T, et al. Factors affecting survival after resection of intrahepatic cholangiocarcinoma. Surg Today 2014;44:1847-54. [PubMed]
- Puhalla H, Schuell B, Pokorny H, et al. Treatment and outcome of intrahepatic cholangiocellular carcinoma. Am J Surg 2005;189:173-7. [PubMed]
- Valverde A, Bonhomme N, Farges O, et al. Resection of intrahepatic cholangiocarcinoma: a Western experience. J Hepatobiliary Pancreat Surg 1999;6:122-7. [PubMed]
- Takada Y, Ito T, Ueda M, et al. Living donor liver transplantation for patients with HCC exceeding the Milan criteria: a proposal of expanded criteria. Dig Dis 2007;25:299-302. [PubMed]
- Kwon CH, Kim DJ, Han YS, et al. HCC in living donor liver transplantation: can we expand the Milan criteria? Dig Dis 2007;25:313-9. [PubMed]
- Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-32. [PubMed]
- Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;10:35-43. [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [PubMed]
- Pichlmayr R, Lamesch P, Weimann A, et al. Surgical treatment of cholangiocellular carcinoma. World J Surg 1995;19:83-8. [PubMed]
- Casavilla FA, Marsh JW, Iwatsuki S, et al. Hepatic resection and transplantation for peripheral cholangiocarcinoma. J Am Coll Surg 1997;185:429-36. [PubMed]
- Pascher A, Jonas S, Neuhaus P. Intrahepatic cholangiocarcinoma: indication for transplantation. J Hepatobiliary Pancreat Surg 2003;10:282-7. [PubMed]
- Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation 2000;69:1633-7. [PubMed]
- Hong JC, Jones CM, Duffy JP, et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg 2011;146:683-9. [PubMed]
- Hyder O, Marsh JW, Salem R, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol 2013;20:3779-86. [PubMed]
- Kuhlmann JB, Euringer W, Spangenberg HC, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol 2012;24:437-43. [PubMed]
- Ibrahim SM, Mulcahy MF, Lewandowski RJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer 2008;113:2119-28. [PubMed]
- Zeng ZC, Tang ZY, Fan J, et al. Consideration of the role of radiotherapy for unresectable intrahepatic cholangiocarcinoma: a retrospective analysis of 75 patients. Cancer J 2006;12:113-22. [PubMed]
- Lo SS, Dawson LA, Kim EY, et al. Stereotactic body radiation therapy for hepatocellular carcinoma. Discov Med 2010;9:404-10. [PubMed]
- Ackerman NB. The blood supply of experimental liver metastases. IV. Changes in vascularity with increasing tumor growth. Surgery 1974;75:589-96. [PubMed]
- Shinohara ET, Mitra N, Guo M, et al. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2008;72:1495-501. [PubMed]
- Dawson LA, Eccles C, Bissonnette JP, et al. Accuracy of daily image guidance for hypofractionated liver radiotherapy with active breathing control. Int J Radiat Oncol Biol Phys 2005;62:1247-52. [PubMed]
- Wehbe H, Henson R, Meng F, et al. Interleukin-6 contributes to growth in cholangiocarcinoma cells by aberrant promoter methylation and gene expression. Cancer Res 2006;66:10517-24. [PubMed]
- Fava G, Alpini G, Rychlicki C, et al. Leptin enhances cholangiocarcinoma cell growth. Cancer Res 2008;68:6752-61. [PubMed]
- Raderer M, Hejna MH, Valencak JB, et al. Two consecutive phase II studies of 5-fluorouracil/leucovorin/mitomycin C and of gemcitabine in patients with advanced biliary cancer. Oncology 1999;56:177-80. [PubMed]
- Gebbia V, Giuliani F, Maiello E, et al. Treatment of inoperable and/or metastatic biliary tree carcinomas with single-agent gemcitabine or in combination with levofolinic acid and infusional fluorouracil: Results of a multicenter phase II study. J Clin Oncol 2001;19:4089-91. [PubMed]
- Kubicka S, Rudolph KL, Tietze MK, et al. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepatogastroenterology 2001;48:783-9. [PubMed]
- Penz M, Kornek GV, Raderer M, et al. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann Oncol 2001;12:183-6. [PubMed]
- Valencak J, Kornek GV, Raderer M, et al. Gemcitabine for the Treatment of Advanced Biliary Tract Carcinomas: Evaluation of Two Different Dose Regimens. Onkologie 1999;22:498-501.
- Mezger J, Sauerbruch T, Ko Y, et al. Phase II Study with Gemcitabine in Gallbladder and Biliary Tract Carcinomasa. Onkologie 1998;21:232-34.
- Kim ST, Park JO, Lee J, et al. A phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer 2006;106:1339-46. [PubMed]
- André T, Tournigand C, Rosmorduc O, et al. Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol 2004;15:1339-43. [PubMed]
- Gebbia N, Verderame F, Di Leo R, et al. A phase II study of oxaliplatin (O) and gemcitabine (G) first line chemotherapy in patients with advanced biliary tract cancers. J Clin Oncol 2005;23:abstr 4132.
- Nakazawa K, Dobashi Y, Suzuki S, et al. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J Pathol 2005;206:356-65. [PubMed]
- Ramanathan RK, Belani CP, Singh DA, et al. Phase II study of lapatinib, a dual inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase 1 and 2 (Her/2-neu) in patients (pts) with advanced biliary tree cancer (BTC) or hepatocellular cancer (HCC). A California consortium (CCC-P) trial. J Clin Oncol 2006;24:abstr 4010.
- Clark JW, Meyerhardt JA, Sahani DV, et al. Phase II study of gemcitabine, oxaliplatin in combination with bevacizumab (GEMOX-B) in patients with unresectable or metastatic biliary tract and gallbladder cancers. J Clin Oncol 2007;25:abstr 4625.
- El-Khoueiry AB, Rankin C, Lenz HJ, et al. A phase II study of sorafenib (BAY 43–9006) as single agent in patients (pts) with unresectable or metastatic gallbladder cancer or cholangiocarcinomas. J Clin Oncol 2007;25:abstr 4639.


