The roles of miRNA-143 in colon cancer and therapeutic implications
Abstract
Activation of multiple signal pathways is necessary for cancer initiation, maintenance and metastasis. MiRNA-143 can regulate several signal pathways and decreased miRNA-143 is associated with many cancers including colon cancer. In this review, we summarise the role of miRNA-143 in colon cancer and therapeutic implications. Several studies have shown that miRNA-143 is reduced in colon cancer. This could result in activation of multiple signal pathways including PI3K/Akt, MAPK and HGF/MET as miRNA-143 acts on signal molecules Ras, Erk5, Akt and MCC1 to reduce their translation. Introduction of miRNA-143 into colon cancer cells induced apoptosis and slower growth in a xenograft model. Therefore miRNA-143 could be developed for the treatment of colon cancer. Molecular markers to identify the suitable cases for the implication of miRNA-143 may be necessary to increase the response rate. We propose that obesity-associated cancer could be a good candidate as it is characterised by activation of multiple signal pathways that are targets of miRNA-143.
Key words
Colon cancer; obesity-associated cancer; miRNA-143
Introduction
Colon cancer is the second most common cancer in males and third in females according to a recent epidemiological study (1). The 5 year survival rate of colon cancer has less than 10% if the cancer has metastasized (2). Both environmental factors and genetic defects have been identified to be involved in the initiation and progression of the disease. The environmental factors include obesity and dietary factors such as intake of red meat, high fat and alcohol (3). The genetic defects include activating mutations of oncogenes such as K-Ras, Cox-2, EGFR, PTEN and PIK3CA and inactivating mutations of tumor suppressor genes such as APC, TP53 and TGF-β (4,5). These factors can alter multiple intracellular signal pathways which in turn decrease apoptosis and increase cell proliferation to cause cancer (4,6).
Recently microRNAs (miRNAs), which are noncoding, single stranded small RNAs, have been found to act on a large number of human genes as a finetune regulation mechanism of gene expression (7,8). MiRNAs bind to 3-untranslational regions (3-UTR) of mRNAs to decrease translation to regulate cellular processes such as proliferation and apoptosis. Therefore, it is not surprising that dysregulation of miRNAs have been found in many cancers and are associated with carcinogenesis and cancer treatment responses (9-11). MiRNA-143 is a tumour suppressor and has been found to be reduced in many types of cancer cells including colon, gastric, ovarian, esophageal, bladder cancers, osteosarcoma, liposarcoma and B-cell leukemia (12-20). This reduction is associated with increased incidence of these cancers and the expression of miRNA-143 in organs can prevent carcinogenesis (18,21). MiRNA-143 affects cancer development through its inhibitory effect on multiple signal pathways such as mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) and hepatocyte growth factor/Met tyrosine kinase (HGF/Met). Therefore, introduction of miRNA-143 into cancer cells could be a potential approach for the treatment of cancer. In this review we summarise the role of miRNA-143 in colon cancer and therapeutic implications, especially in obesity-associated colon cancer, which is difficult to treat due to activation of multiple signal pathways.
MiRNA-143 in colon cancer
MiRNA-143 is found to be down-regulated in colon cancer (12,18,19,22). Gao et al. showed that miRNA-143 is four fold less in colon cancer compared to surrounding tissues (18). The decreased levels of miRNA-143 could lead to activation of survival signal pathways and increase carcinogenesis. For example, miRNA-143 has been demonstrated to mediate ecotropic viral integration site 1 oncoprotein (Evi 1) to-cause colon cance (18). Evi is a retroviral gene inserted into human genome and its encoding protein can interact with miRNA-143 to downregulate miRNA-143, resulting in activation of PI3K/Akt pathway and decreased apoptosis (23).
MiRNA-143 is also involved in colon cancer progression and decreased miRNA-143 is a marker for poor prognosis of colon cancer (24). MiRNA-143 is associated with both metastasis of the colon cancer and drug resistance to chemotherapy. It has been demonstrated that introduction of miRNA-143 into colon cancer cell line SW620 decreased cell migration and invasion, indicating its important role in colon cancer metastasis (25). Introduction of miRNA-143 into HCT116 also increased their sensitivity to 5-FU (26). Decreased miRNA-143 is correlated with decreased progression-free survival in colon cancer patients treated with anti-EGFR therapy, which is commonly used in colon cancer patients with wild-type of K-ras (24,27).
Siganal molecules targeted by miRNA-143
MiRNA-143 has been demonstrated to decrease protein translation of several signal molecules including Rat Sarcoma protein (Ras), Extracellular signal-regulated protein kinase 5 (Erk5) and Akt and Metastasis-associated in colon cancer-1 (MACC1) (Figure 1). The decreased expression of these proteins by miRNA-143 results in decreased activities of MAPK, PI3K/Akt and HGF/Met pathways which play key roles in cancer progression.
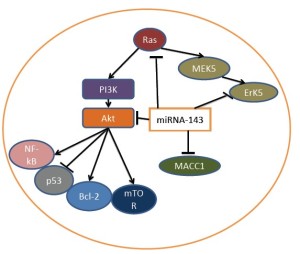
Ras
Ras is a major oncogene and its activation can increase carcinogenesis, cancer progression and metastasis (28,29). There are three isoforms of Ras: K-Ras, H-Ras and N-Ras. K-ras is mutated in a subset of colon cancers, leading activation of its downstream signalling pathways including PI3K/Akt and MAPK pathways (28). A recent study showed that inhibition of K-ras by siRNA also lead to decreased Wnt signalling pathway (30). Ras can increase BMP signalling, which causes TAK1 (MAP3K7) activation and increases Wnt pathway. Activating mutation of K-ras is well known to cause the drug resistance to EGFR inhibitors and thus affects treatment outcome (27). MiRNA-143 has been shown to target Ras and inhibit the activity of MAPK pathway (18). Five miRNA-143 binding sites have been identified in the 3-UTR of Ras and vilidated by luciferase assay, in which a reporter protein is used to indicate the function of 3-UTR of ras (18).
Erk5
Erk5 is a member of MAPK family, which also includes ERK1/2, c-JUN and p38 MAPK (31-33) Erk5 is activated in response to growth factor stimulation or environmental stresses to mediate cell proliferation and migration (34). The biological effect of Erk5 is mediated by several downstream target proteins including myc and sap 1 (35). Erk5 is involved in cancer initiation and metastasis and inhibition of Erk5 by small molecules has therapeutic effect (34,36-38). MiRNA-143 has been demonstrated to decrease Erk5 protein translation (39,40). There are inverse relationships between miRNA-143 and Erk5 in both cancer tissues and cancer cell lines (40). Introduction of MiRNA-143 into cancer cells reduced Erk5 expression. This is further demonstrated by luciferase assay and introduction of the mutation in the 3-UTR of Erk5 abolished the effect of miRNA-143 (40).
Akt
PI3K/Akt is a survival pathway and plays an important role in cell proliferation, differentiation and apoptosis. Its abnormal activation has been found in many cancers including colon cancer (41,42). Inhibition of the PI3K/Akt pathway has been implicated in cancer therapy (43-46). Akt is a major component of the PI3K/Akt pathway mediating most biological effects of PI3K through a broad range of downstream target proteins (20). MiRNA-143 has been shown to decrease the level of Akt at translational level (39). Introduction of miRNA-143 into T24 cancer cells resulted in decreased levels of Akt protein but not mRNA.
MACC1
Metastasis-associated in colon cancer-1 (MACC1) was identified in 2009 as a gene involved in the metastasis of colon cancer (47,48). MACC1 regulates HGF-MET signalling pathway, which is known to promote colon cancer proliferation and invasion (49,50). MACC1 may also regulate other proteins to increase colon cancer progression (51). In cancer patients, increased levels of MACC1 in colon cancer has been shown to decrease survival rate (52,53). In mouse models shRNA against MACC1 reduced tumor growth and metastasis(54). MiRNA-143 can target MACC1 to decrease its expression level (25). The direct interaction of miRNA-143 and MACC1 has been demonstrated by luciferase assay (25).
Therapeutic implications
The therapeutic effect of miRNA-143 has been tested in both cancer cell lines and an animal model. A study showed that the introduction of miRNA-143 in the colon cancer cell line HCT116 lead to decreased proliferation and increased apoptosis (55). Transfection of miRNA-143 into colon cancer cell lines DLD-1 and SW480 inhibited the growth of these cells (12). In animal model, the HCT-116 cells with over-expression of miRNA-143 grew slower in the xenografted mice than control HCT116 cells, indicating the cancer cells were weakened by the introduction of miRNA-143. MiRNA-143 was also shown to decrease colon cancer cell invasion and metastasis (25).
The effect of miRNA-143 has been tested in combination with chemotherapeutic agents. It has been shown that addition of miRNA-143 increased the effect of 5-FU in colon cancer cell line (26). The miRNA-143 has been shown to have an additive effect with cisplatin in the bladder cancer cell line T24 cells (39). It will be interesting to test if miRNA-143 increases the effectiveness of oxaliplatin which is also a first line anticolon cancer drug (56).
The possible implications for obesity-associated colon cancer
Obesity has been associated with many cancers and affects the incidence, metastasis and therapeutic outcome of colon cancer (57). The main cause for the association of obesity and colon cancer is the increase of cancer risk factors in obesity such as insulin/IGF1, leptin, adiponection, estrogen and IL-6, IL-17 and TNF-alpha (57,58). These factors play important roles in cancer by activating multiple signal pathways including PI3K/Akt, MAPK and Stat3 pathways (56-61). Activation of these signal pathways can increase carcinogenesis, metastasis and drug resistance (62,63). Therefore, inhibition of these pathways has preventive and therapeutic implications (46,64). A recent study showed that high-fat diet-induced obesity increased azoxymethane induced colon polyps by two-folds and the activation of Akt and MAPK pathways in the early stage is critical for the carcinogenesis (65). In animal experiments, inhibition of the PI3K/Akt pathway by small molecules or reduction of PI3K/Akt through food restriction can abolish the obesityincreased colon cancer incidence (66,67). As miRNA-143 can inhibit several pathways activated in obesity-associated colon cancer, it therefore could be a good candidate for prevention or treatment of obesity-associated colon cancer. It will be interest to investigate the effect of miRNA-143 for drug resistance in obesity-associated colon cancer.
Potential side effects, such as unwanted insulin resistance that can be caused by increased miR-143, have to be minimised. The PI3K/Akt pathway is also important for insulin signalling in glucose metabolism, and inhibition of the pathway could lead to increased insulin resistance. Jordan et al. found that miRNA-143 is increased in the livers in obese mice resulting in reduced activity of the Akt pathway (68). This effect of miRNA-143 has been regarded as a cause for insulin resistance in obesity. A recent paper has shown that orally administered miRNA-143 could be stable and may also enter the blood stream (69). Therefore, when introducing miRNA-143 to prevent and treat obesityassociated colon cancer, the miRNA-143 may enter the circulation system to affect the function of Akt required for the regulation of glucose homeostasis. Preventing miRNA-143 from leaking into the circulation system could be the key for the successful implication of miRNA-143 in treating obesity-associated colon cancer. This could be achieved by introducing synthetic miRNA-143 specifically in colon cancer cells instead of delivering systemically.
Conclusions
MiRNA-143 can decrease translation of multiple signal molecules to regulate MAPK, PI3K/Akt and HGF/Met pathways. Decreased levels of miRNA-143 in colon cancer at least partially account for increased activation of these pathways. Therefore, introduction of miRNA to cancer cells may be used for treatment of the disease. Obesity-associated colon cancer could be a good candidate for the implication of miRNA-143 as multiple signal pathways including PI3K and MAPK are activated in obesity-associated colon cancer and cause the resistance to chemotherapeutic treatment, MiRNA-143 could be delivered to the site of colon cancer after oral administration. However, it could enter into adipose tissue via blood and cause insulin-resistance. This side effect needs to be minimised, probably by specific delivery of miRNA-143 to colon cancer cells.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Desantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;64:220-41.
- Chen J. Is Src the key to understanding metastasis and developing new treatments for colon cancer? Nat Clin Pract Gastroenterol Hepatol 2008;5:306-7.
- Watson AJ, Collins PD. Colon cancer: A civilization disorder. Dig Dis 2011;29:222-8.
- Chen J, Huang XF. The signal pathways in azoxymethaneinduced colon cancer and preventive implications. Cancer Biol Ther 2009;8:1313-7.
- Zhu H, Dougherty U, Robinson V, et al. EGFR signals downregulate tumor suppressors miR-143 and miR-145 in Western diet-promoted murine colon cancer: role of G1 regulators. Mol Cancer Res 2011;9:960-75.
- Chen J, Huang XF. Activation of p53 for the treatment of cancer. J Cell Biochem 2009;107:567-8.
- Okada H, Kohanbash G, Lotze MT. MicroRNAs in immune regulation-opportunities for cancer immunotherapy. Int J Biochem Cell Biol 2010;42:1256-61.
- Eamens AL, Agius C, Smith NA, et al. Efficient silencing of endogenous microRNAs using artificial microRNAs in Arabidopsis thaliana. Mol Plant 2011;4:157-70.
- Che X, Huang C. microRNA, Cancer and Cancer Chemoprevention. Curr Mol Pharmacol 2012. [Epub ahead of print].
- Kong YW, Ferland-McCollough D, Jackson TJ, et al. microRNAs in cancer management. Lancet Oncol 2012;13:e249-58.
- Xiao Y, Guan J, Ping Y, et al. Prioritizing cancer-related key miRNA-target interactions by integrative genomics. Nucleic Acids Res 2012. [Epub ahead of print].
- Akao Y, Nakagawa Y, Naoe T. MicroRNAs 143 and 145 are possible common onco-microRNAs in human cancers. Oncol Rep 2006;16:845-50.
- Takagi T, Iio A, Nakagawa Y, et al. Decreased expression of microRNA-143 and -145 in human gastric cancers. Oncology 2009;77:12-21.
- Akao Y, Nakagawa Y, Iio A, et al. Role of microRNA-143 in Fas-mediated apoptosis in human T-cell leukemia Jurkat cells. Leuk Res 2009;33:1530-8.
- Osaki M, Takeshita F, Sugimoto Y, et al. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol Ther 2011;19:1123-30.
- Ugras S, Brill E, Jacobsen A, et al. Small RNA sequencing and functional characterization reveals MicroRNA-143 tumor suppressor activity in liposarcoma. Cancer Res 2011;71:5659-69.
- Zhang H, Cai X, Wang Y, et al. microRNA-143, downregulated in osteosarcoma, promotes apoptosis and suppresses tumorigenicity by targeting Bcl-2. Oncol Rep 2010;24:1363-9.
- Gao JS, Zhang Y, Tang X, et al. The Evi1, microRNA-143, K-Ras axis in colon cancer. FEBS Lett 2011;585:693-9.
- Akao Y, Nakagawa Y, Naoe T. MicroRNA-143 and -145 in colon cancer. DNA Cell Biol 2007;26:311-20.
- Nakagawa Y, Iinuma M, Naoe T, et al. Characterized mechanism of alpha-mangostin-induced cell death: caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR- 143 expression in human colorectal cancer DLD-1 cells. Bioorg Med Chem 2007;15:5620-8.
- Lin T, Dong W, Huang J, et al. MicroRNA-143 as a tumor suppressor for bladder cancer. J Urol 2009;181:1372-80.
- Akao Y, Nakagawa Y, Kitade Y, et al. Downregulation of microRNAs-143 and -145 in B-cell malignancies. Cancer Sci 2007;98:1914-20.
- Liu Y, Chen L, Ko TC, et al. Evi1 is a survival factor which conveys resistance to both TGFbeta- and taxol-mediated cell death via PI3K/AKT. Oncogene 2006;25:3565-75.
- Pichler M, Winter E, Stotz M, et al. Down-regulation of KRAS-interacting miRNA-143 predicts poor prognosis but not response to EGFR-targeted agents in colorectal cancer. Br J Cancer 2012. [Epub ahead of print].
- Zhang Y, Wang Z, Chen M, et al. MicroRNA-143 targets MACC1 to inhibit cell invasion and migration in colorectal cancer. Mol Cancer 2012;11:23.
- Borralho PM, Kren BT, Castro RE, et al. MicroRNA-143 reduces viability and increases sensitivity to 5-fluorouracil in HCT116 human colorectal cancer cells. FEBS J 2009;276:6689-700.
- Chen J, Huang XF, Katsifis A. Activation of signal pathways and the resistance to anti-EGFR treatment in colorectal cancer. J Cell Biochem 2010;111:1082-6.
- Duhamel S, Hébert J, Gaboury L, et al. Sef downregulation by Ras causes MEK1/2 to become aberrantly nuclear localized leading to polyploidy and neoplastic transformation. Cancer Res 2012;72:626-35.
- Makrodouli E, Oikonomou E, Koc M, et al. BRAF and RAS oncogenes regulate Rho GTPase pathways to mediate migration and invasion properties in human colon cancer cells: a comparative study. Mol Cancer 2011;10:118.
- Singh A, Sweeney MF, Yu M, et al. TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. Cell 2012;148:639-50.
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 2001;410:37-40.
- Dhanasekaran DN, Johnson GL. MAPKs: function, regulation, role in cancer and therapeutic targeting. Oncogene 2007;26:3097-9.
- Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 2007;1773:1358-75.
- Lochhead PA, Gilley R, Cook SJ. ERK5 and its role in tumour development. Biochem Soc Trans 2012;40:251-6.
- Wang X, Tournier C. Regulation of cellular functions by the ERK5 signalling pathway. Cell Signal 2006;18:753-60.
- Drew BA, Burow ME, Beckman BS. MEK5/ERK5 pathway: the first fifteen years. Biochim Biophys Acta 2012;1825:37-48.
- Ramsay AK, McCracken SR, Soofi M, et al. ERK5 signalling in prostate cancer promotes an invasive phenotype. Br J Cancer 2011;104:664-72.
- Razumovskaya E, Sun J, Rönnstrand L. Inhibition of MEK5 by BIX02188 induces apoptosis in cells expressing the oncogenic mutant FLT3-ITD. Biochem Biophys Res Commun 2011;412:307-12.
- Noguchi S, Mori T, Hoshino Y, et al. MicroRNA-143 functions as a tumor suppressor in human bladder cancer T24 cells. Cancer Lett 2011;307:211-20
- Clapé C, Fritz V, Henriquet C, et al. miR-143 interferes with ERK5 signaling, and abrogates prostate cancer progression in mice. PLoS One 2009;4:e7542.
- Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol 2010;7:209-19.
- Liu P, Cheng H, Roberts TM, et al. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov 2009;8:627-44.
- Bartholomeusz C, Gonzalez-Angulo AM. Targeting the PI3K signaling pathway in cancer therapy. Expert Opin Ther Targets 2012;16:121-30.
- Polak R, Buitenhuis M. The PI3K/PKB signaling module as key regulator of hematopoiesis: implications for therapeutic strategies in leukemia. Blood 2012;119:911-23.
- Sheppard K, Kinross KM, Solomon B, et al. Targeting PI3 kinase/AKT/mTOR signaling in cancer. Crit Rev Oncog 2012;17:69-95.
- Chen J. Targeted therapy of obesity-associated colon cancer Transl Gastrointest Cancer 2012;1:44-57.
- Stein U, Walther W, Arlt F, et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med 2009;15:59-67.
- Arlt F, Stein U. Colon cancer metastasis: MACC1 and Met as metastatic pacemakers. Int J Biochem Cell Biol 2009;41:2356-9.
- Boardman LA. Overexpression of MACC1 leads to downstream activation of HGF/MET and potentiates metastasis and recurrence of colorectal cancer. Genome Med 2009;1:36.
- Stein U, Smith J, Walther W, et al. MACC1 controls Met: what a difference an Sp1 site makes. Cell Cycle 2009;8:2467-9.
- Galimi F, Torti D, Sassi F, et al. Genetic and expression analysis of MET, MACC1, and HGF in metastatic colorectal cancer: response to met inhibition in patient xenografts and pathologic correlations. Clin Cancer Res. 2011;17:3146-56.
- Lang AH, Geller-Rhomberg S, Winder T, et al. A common variant of the MACC1 gene is significantly associated with overall survival in colorectal cancer patients. BMC Cancer 2012;12:20.
- Shirahata A, Shinmura K, Kitamura Y, et al. MACC1 as a marker for advanced colorectal carcinoma. Anticancer Res 2010;30:2689-92.
- Pichorner A, Sack U, Kobelt D, et al. In vivo imaging of colorectal cancer growth and metastasis by targeting MACC1 with shRNA in xenografted mice. Clin Exp Metastasis 2012;29:573-83.
- Borralho PM, Simões AE, Gomes SE, et al. miR-143 overexpression impairs growth of human colon carcinoma xenografts in mice with induction of apoptosis and inhibition of proliferation. PLoS One 2011;6:e23787.
- Chen J, Huang XF, Qiao L, et al. Insulin caused drug resistance to oxaliplatin in colon cancer cell HT29. J Gastrointest Oncol 2011;2:27-33.
- Chen J. Multiple signal pathways in obesity-associated cancer. Obes Rev 2011;12:1063-70.
- Chen J. Multiple signal pathways in obesity-associated skin cancer. Toxicol Appl Pharmacol 2010;247:166; author reply 167.
- Chen J. The Src/PI3K/Akt pathway may play a key role in the production of IL-17 in obesity. J Leukoc Biol 2010; 87:355; author reply 357.
- Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer 2011;11:886-95.
- Johnson C, Han Y, Hughart N, et al. Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl Gastrointest Cancer 2012;1:58-70.
- Chen J.The Src/PI3K/Akt signal pathway may play a key role in decreased drug efficacy in obesity-associated cancer. J Cell Biochem 2010;110:279-80.
- Chen J, Katsifis A, Hu C, et al. Insulin decreases therapeutic efficacy in colon cancer cell line HT29 via the activation of the PI3K/Akt pathway. Curr Drug Discov Technol 2011;8:119-25.
- Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev 2009;10:610-6.
- Park SY, Kim JS, Seo YR, et al. Effects of diet-induced obesity on colitis-associated colon tumor formation in A/J mice. Int J Obes (Lond) 2012;36:273-80.
- Algire C, Amrein L, Zakikhani M, et al. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocr Relat Cancer 2010;17:351-60.
- Moore T, Beltran L, Carbajal S, et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila) 2008;1:65-76.
- Jordan SD, Krüger M, Willmes DM, et al. Obesityinduced overexpression of miRNA-143 inhibits insulinstimulated AKT activation and impairs glucose metabolism. Nat Cell Biol 2011;13:434-46.
- Zhang L, Hou D, Chen X, et al. Exogenous plant MIR168a specifically targets mammalian LDLRAP1: evidence of cross-kingdom regulation by microRNA. Cell Res 2012;22:107-26.


