Distribution and expression of the ERM family members, ezrin, radixin, moesin and EHM2 in human colon cancer and the clinical relevance
Introduction: The Ezrin-Moesin-Radixin (ERM) family is a protein family that are cytoskeletal linker
proteins and acts as an anchorage protein for cell surface molecules and regulates cell adhesion in epithelial
cells. This study investigated the expression pattern of the ERM family and its associated member, EHM2, in
human colon tissues (both normal and tumour) at the protein and messenger levels and explored the clinical
and prognostic values of the family.
Methods: Fresh frozen normal colon and colon cancer tissues were used. The distribution of ezrin
protein in the tissues was investigated using immunofluorescence (IFC). Transcript levels of ezrin, moesin,
radixin and EHM2 were determined using quantitative polymerase chain reaction (PCR) analysis. Levels of
expression were analysed against tumour staging and clinical outcome of the patients.
Results: In normal tissues, ezrin stained strongly in the region of intercellular adhesions and wasabsent
in the apical region of the colonic epithelial cells. There was little cytoplasmic staining in normal cells.
However, in tumour tissues, colon cancer cells showed a shift in the pattern of staining in which ezrin stained
strongly in the cytoplasmic area of cancer cells, whereas the inter-cellular staining was either absent or weak.
Using quantitative analysis, it was found that there was a marked increase in the levels of ezrin, moesin and
radixin in tumour tissues compared with normal tissues However, in advanced disease, both Dukes C and
Dukes B tumours had significantly higher levels of ezrin transcript compared with Dukes A tumours (P=0.0047
and P=0.0004, respectively). High levels of ezrin expression were also significantly associated with disease
free and overall survival of the patients (P=0.006 and P=0.011, respectively).
Conclusions: In normal epithelial cells, ezrin is confined to the cell-cell junction area and confers cellcell
adhesion. In colon cancer cells, ezrin is redistributed to the cytoplasmic region, which may weaken the
adhesion between cells and increase invasive potential. Together with an increase in mRNA expression in late
stage tumours, it is concluded that the loss of ezrin at the inter-cellular region and the rise in the cytoplasmic
levels of ezrin in colon cancer cells is linked to the aggressiveness of colon cancer cells and the clinical
outcome of the patients.
Key words: ERM family; ezrin; moesin; radixin; EHM2; cell adhesion; cytoskeletal proteins; colon cancer; Dukes staging
Introduction
ERM (ezrin, moesin and radixin) belongs to a larger protein family, known as FERM (4.1 protein, Ezrin, Radixin, Moesin) domain which functions as a protein docking surface with the cytosolic tail of transmembrane proteins such as CD44 (1-4). These proteins are cytoskeleton associated and have traditionally been known as molecules involved in maintaining the integrity and morphology of cells. These proteins also been widely shown to regulate the migration of the cells, and are key to the organsing the ruffling of the membrane. They have also been suggested to be candidate molecules in directional cell movement including that of cancer cells (5,6). The FERM proteins are characterised by the presence of the FERM domain, 300 amino acids in length. The FERM domain consists of three subdomains that fold independently but are closely associated with one another and form a cloverleaf with lobes of approximately 100 amino acids each (7,8). The FERM domain can interact with numerous protein-binding partners, including transmembrane ion channels and adhesion molecules [e.g., intercellular adhesion molecule (ICAM), CD44] (9-12). It also interacts with other cytoplasmic proteins such as calmodulin and membrane-associated guanylate kinases (MAGUKs) (13). Presently, approximately 50 FERM proteins have been identified and can be further divided into Merlin/ERM (Ezrin, Radixin and Moesin) family, Protein 4.1 family, NBL4 family (including EHM2), Talin family, protein tyrosine phosphase H1 (PTPH1) family and Tyrosin kinase family.
The main functions of the ERM family proteins are thought to be acting as protein linkers which link the cell membrane to the cytoskeletal proteins, cell surface molecules (cell adhesion molecules) to the cytoskeleton and, by doing so regulating cell adhesion and migration. They have also been implicated in cell-cell communication, apoptosis, carcinogenesis and metastasis. For example, Ezrin has been shown to act as an anchorage protein for CD44, a cell adhesion molecule that is widely involved in metastatic cancer cells. It also acts as an anchorage protein for ICAM2. In the last two decades, the clinical links between the ERM family and cancer have been reported in a variety of human cancers. Most of the clincical studies have focused on ezrin. High levels of ezrin in glioma are associated with shorter survival and disease progression (14). Smilar findings have been reported in prostate cancer, lung cancer, and most widely in soft tissue sarcomas (15-20). Ezrin has also been shown to co-operate with c-Src in mammary cancer cells and regulate cell-cell contact and migration (21). In osteosacoma and other soft tissue derived malignancies, including malignant melanoma and rhabdomyosarcoma, ezrin has been found to be key to the metastatic potential of tumour cells (17,22-24). However, the link between ezrin and cancer is not without controversy. In serous ovarian cancer, reduction or loss of ezrin has been reported to be linked to shorter survival (24). In breast cancer, this link appears to be less clear, while the transcript levels of ezrin are lower in tumours (25), the cellular location of the protein was thought to be key (26). In pancreatic cancer, poorly differentiated pancreatic ductal adenocarcinoam showed more membranous ezxrin staining compared with well differentiated tumours (27).
Studies on moesin and radixin are rather limited. In an early report, it has been shown that moesin protein is linked to lymph node metastasis and invasion in oral squamous cell carcinoma (28). The cytoplasmic expression of moesin was thought to be a feature of metastatic cells in the lymph nodes in these patients. Investigations into other members of the FERM family are reported. For example, one of the few reports for EHM2 came from a study on breast cancer, in which EHM2 protein and transcripts are signficantly raised in breast tumour tissues compared with normal tissues and the raised levels are linked to shorter disease free and overall survival of the patients (29). In vitro, levels of EHM2 have been shown to be associated with invasion and migration, via mechansims such as upregulation of metalloproteinase-9 (MMP-9) (29).
Despite the recognition of the functional roles of ERM family proteins, the impact of these molecules in clinical cancer remains controversial. In the current study, we have examined the distribution of ezrin in human colon tissues and colon cancer, and correlated the levels of expression of ezrin with disease progression.
Materials and methods
Cells and tissue samples
Fresh colon tumour tissues (n=94) and normal background tissues from the same patients (n=80) were collected immediately after surgery and stored in the deep freezer until use. Details of histology and clinical outcome were obtained from Pathology reports (Table 1). Ethical approval and patient consent were obtained. Patients were routinely followed clinically after surgery. The median followup period was 63 (IQR 8-176) months.
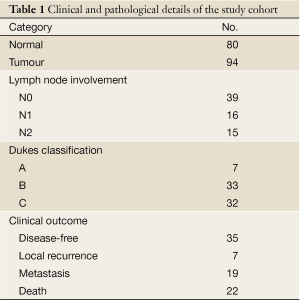
Full table
Materials
RNA extraction kit and RT kit were obtained from Sigma-Aldrich (Poole, Dorset, England). PCR primers were designed using Beacon Designer (California, USA) and synthesised by SigmaGenesis. Master mix for routine PCR and customised master mix for quantitative PCR were from BioRad (Hemel Hemstead, England). Polyclonal antibody to human moesin and radixin and monoclonal antibody to human ezrin were purchased from Santa-Cruz Biotechnologies Ltd (Santa Cruz, California, USA) and Affinity Antibodies Inc (Exeter, England, UK), respectively. FITC, TRITC-conjugated secondary antibodies were from Sigma-Aldrich (Poole, Dorset, England, UKL).
Immunofluorescent staining of ezrin
This was based on the method we recently described (30,31). Frozen sections of colon cancer and normal colon tissues were cut at a thickness of 6µm using a cryostat. The sections were mounted on super frost plus microscope slides, air dried and then fixed in a mixture of 50% Acetone and 50% methanol. The staining procedure was completed simultaneously for all the section, to ensure comparison. The sections were first rehydrated and then incubated for 20 mins in a blocking bufber which had 10% horse serum. The slides were probed with the primary antibody (diluted 1:100 for anti-moesin and anti-radixin and 1:50 for anti-ezrin). Following extensive washings, sections were incubated for 30 mins in the secondary FITC- or TRITCconjugated antibody. The slides were then mounted using FluoSaveTM mounting media and stored in the dark at 4 ℃. Staining was exmined using a fluorescent microscope (Olympus) and photographed using a digital camera (Hamamatsu).
Tissue processing, RNA extraction, cDNA synthesis, and RT-PCR
15-20 frozen sections were mixed and homogenised using a hand-held homogeniser, in ice cold RNA extraction solution. The concentration of RNA was determined using a UV spectrophotometer. Reverse transcription was performed using a RT kit with an anchored oligo-dt primer supplied by AbGene, using 1 g total RNA in a 96-well plate. The quality of cDNA was verified using -actin primers.
Quantitative analysis of gene transcripts
The levels of ezrin, moesin, radixin and EHM2 transcripts from the above-prepared cDNA was determined using a real-time quantitative PCR, based on the AmplifluorTM technology as we recently reported (25,32), modified from a method previous reported (33). Briefly, pairs of PCR primers were designed using the Beacon Designer software (version 2, California, USA) (sequence given in Table 2), but to one of the primers, an additional sequence, known as the Z sequence (5'actgaacctgaccgtaca'3) which is complementary to the universal Z probe (Intergen Inc., England, UK), was added (Table 2). The reaction was carried out using the following: 10 pmol of specific forward primer, 1 pmol reverse primer which has the Z sequence, 10 pmol of FAM-tagged probe (Intergen Inc.), and cDNA from approximately 50 ng RNA. The reaction was carried out using IcyclerIQtm (Bio-Rad) which is equipped with an optical unit that allows real time detection of 96 reactions, using the following conditions: 94 ℃ for 12 minutes, 50 cycles of 94 ℃ for 15 seconds, 55 ℃ for 40 seconds and 72 ℃ for 20 seconds. The levels of the transcripts were generated from a standard that was simultaneously amplified with the samples. Levels of the transcript are shown here in two ways: Ezrin, moesin and radixin transcript and the ratio between the respective ERM members and cytokeratin-19 (CK19), as we have recently reported (32,33).
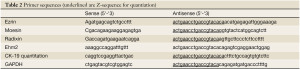
Full table
Statistical analysis was carried out using Mann-Whitney U test and the Kruskal-Wallis test, and Kaplan-Meier survival analysis and Cox proportional analysis, where appropriate, using SPSS (SPAW18) package.
Results
Distribution of Ezrin, moesin and radixin in colon tissesmmary tissues
In normal colon tissues, ezrin staining was seen to be clearly confined to the intercellular region of the adjoining epithelial cells (Figure 1, white arrows). The staining was seen at the luminal end of the crypt as well as the basal part of the epithelial cells. No cytoplasmic staining was seen in epithelial cells. Furthermore, stromal cells had no observed staining. The other striking feature of the staining pattern was that the staining appears to be restricted to the intercellular regions of the epithelial cells (Figure 1, white arrows), the apical regions of the cells were almost completely lack of the staining (Figure 1, red arrows).
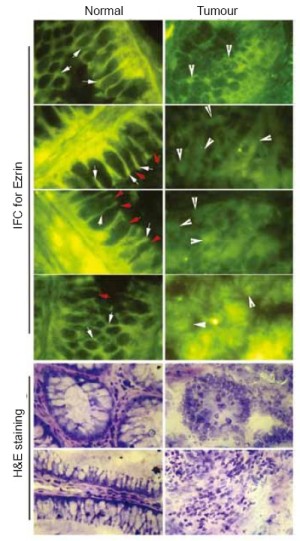
Loss of distribution pattern of ezrin in colon cancer tissues
Colon cancer tissues had a very different staining pattern. As shown in Figure 1, there was strong cytoplasmic staining of ezrin in colon cancer cells. The intercellular staining pattern seen in normal tissues was completely lost.
Correlation between levels of ERM family transcripts and tissue type
We conducted a quantitative analysis of the transcript of the ERM family in human colon rectal tissues. As shown in Table 3, there was a significant higher level of ezrin transcript in colon tumour tissues than normal tissues (P=0.0032). Likewise, levels of moesin and expressed in highly metastatic cells 2 (EHM2) in tumour tissues were also signficantly higher than in normal tissues (Table 3). There does not appear to be a significant difference for radixin in the two types of colon tissues.
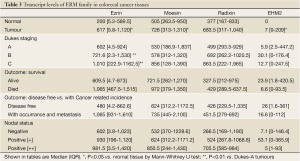
Full table
High levels of ERM transcripts are associated with the disease progression and long term survival
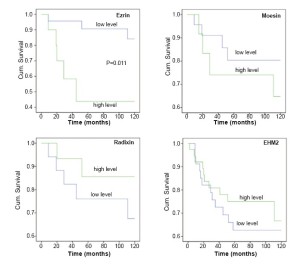
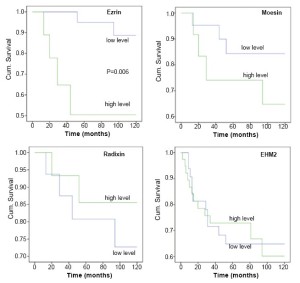
When the levels of ezrin transcript were compared between tumours with different staging, it was revealed that Dukes B and Dukes C tumour had significantly higher levels of the ezrin transcript than Dukes A tumours (P=0.0047 and P=0.0004 B and C vs. A, respectively) (Table 3). Using Kaplan-Meier survival method, it was shown that high levels of ezrin transcript were inversely correlated with shorter overall (P=0.011, Figure 2) and disease free survival (P=0.006, Figure 3). Although levels of Moesin showed a similar trend of inverse relationship with the survivals, this was not statistically signficant. Levels of radixin and EHM2 do not bear a clear relationship with surival.
Discussion
The current study has shown that ezrin, a cytoskelon associated protein has lost its distribution pattern in human colon cancer and that a raised level of ezrin transcript is associated with progression and reduced survival of the patients with colon cancer. Other members of the family, namely moesin, radixin and EHM2, although raised in colon tumours, do not signficantly correlate with survival.
Ezrin has been shown to act as an anchorage protein for cell adhesion molecules, including CD44, ICAM2, and few others. This has been suggested to provide a key mechanism in supporting cell adhesion mediated by these molecules. These adhesion molecules are widely involved in the development and progression of cancers including colon cancer. CD44 has been shown to be over-expressed in human colon cancer (34,35) and is linked to metastasis of tumour cells (36). Colon cancer cell also express ICAMs which may be key to directing the trafficking of itself and other cells (37). Thus, it is suggested that ezrin may act in cells by regulating cell adhesion. Of the adhesion molecules that ezrin is known to support, most are involved in pro-tumour actions such as CD44. Others are tumour suppressors, for example the E-cadherin complex. Results from the current study have shown that ezrin has lost its co-localisation with ICAM2 and that ezrin is relocated to the cytoplasmic region from the intercellular junction area. This suggests that these cellular changes may rendere cancer cells less adhesive and allow them to aquire dissociation properties, thus facilitating there invasiveness.
A number of reports have shown that increased levels of ezrin in tumour are associated with the aggressive nature of the tumours, particularly in neurological tumours (e.g., glioma). The precise mechanisms are not clear, although they can be attributed to the alteration in cell adhesion. The current study has shown that high levels of ezrin transcript in colon cancer cells are also linked to disease progression and reduced long term survival. For example, Dukes B and Dukes C tumours have significantly high levels of ezrin than Dukes A tumours. In rectal cancer, a recent study has shown that high ezrin protein expression is a predictor of the local recurrence (38) and prognosis (39). Taken together, the current study indicates that there is an excessive expression of ezrin in human colon cancer cells and that the over-expressed proteins are primarily cytoplasmic in its location, and not in the cell-cell junction area thus being ‘dis-located’.
It is interesting to observe that the other three members, namely moesin, radixin and EHM2 do not have a clearly relationship with the disease progression. It is clear from the present study that although both moesin and EHM2 are highly expression in colon cancer tissues, the raised levels do not link to the staging and outcome of the patients, a clear contrast to ezrin. The observation on EHM2 is particularly interesting as EHM2 has been shown to be correlated with the outcome in patients with breast cancer (29). Togather, it suggest that Ezrin plays a particular role in colorectal cancer and that other members of the family appear to be less involved in the disease.
In osteosarcoma, a microRNA, miR183, which targets ezrin was reported to be down regulated and that the microRNA, by suppressing ezrin, regulates the aggressiveness of osteosarcoma cells (40). In head and neck squamous cell carcinoma, a microRNA, miTNA133a has been shown similarly to regulate moesin, by inhibiting cell growth and invasion (41). In soft tissue sarcoma, ezrin is a predictor of local recurrence and distant metastasis (42). In breast cancer, three members of the ERM family, namely ezrin, radixin and moesin were found to be reduced in aggressive tumours at transcript level (25). Interestingly, a recent study has shown that the cellular location of ezrin in breast cancer cells is a more useful determinant factor in cell aggressiveness, namely ezrin localised in the apical membrane in normal breast epithelial cell. However, ezrin relocalised to the cytoplasmic region in breast cancer cells (26). The findings from the present study thus suggest that in colorectal cancer, both raised levels of ERM family members, and particularly ezrin and relocation are present.
Together, ezrin, both at protein and transcript levels, appears to be an important prognostic factor for patients with colorectal cancer. It may also allow therapeutic options such as miRNA and siRNA to be developed. No drugs are yet reported to specifically inhibit ezrin, however, some of the epigenetic drugs and estrogen have been shown to upregulate ezrin (43,44). Rational designed compounds that have impact on the activity of ERM have also been recently reported (45).
We propose the following model of action for ezrin in human colon and colon cancer. Firstly, in normal colon epithelial cells, ezrin is co-localised with cell adhesion complexes and governs the adhesiveness and motility of cancer cells. Here, ezrin proteins are exclusively confined to the cell-cell junction area. This is a key feature for the normal epithelial cell. We have previously reported that ezrin interacts with the E-cadherin complex and regulates cell-cell adhesion in colorectal cancer cells (31). For this interaction to take place, the apical membrane location of both proteins is essential. Secondly, in colon cancer cells, the over-expressed ezrin proteins are primarily cytoplasmic. Cells can no longer utilise the cytoplasmic ezrin for the purpose of cell-cell adhesion. Instead, cytoplasmic ezrin may facility the organisation of the cytoskeleton and cell motility. Clearly, a number of questions arise from the current study: how does the over-expression of ezrin occur? Is it translational or transcriptional aberration? Are the over-expressed cytoplasmic proteins functional? How do they regulate the motility of cancer cells? Is the ‘dis-location’ and expression of ezrin in cancers a biological hallmark of cancer aggressiveness?
In conclusion, ezrin over-expression is a frequent phenomenon in colon cancer. The aberration occurs at two levels: at protein level ezrin is seen to be abnormally distributed within the cells and at message level the mRNA levels are over-expressed. In normal epithelial cells, ezrin is confined to the cell-cell junction area and confers cell-cell adhesion. In colon cancer cells, ezrin is redistributed to the cytoplasmic region, which may weaken the adhesion between cells and increase invasive potential. Taking this with an increase in mRNA expression in late stage tumours, it is concluded that the loss of ezrin at the inter-cellular region and the rise in the cytoplasmic levels of ezrin in colon cancer cells is linked to the aggressiveness of human colon cancer.
Acknowledgements
The authors wish to thank Cancer Research Wales and RAF (MLD) for supporting this work.
Disclosure: The author declares no conflict of interest.
References
- Sun CX, Robb VA, Gutmann DH. Protein 4.1 tumor suppressors: getting a FERM grip on growth regulation. J Cell Sci 2002;115:3991-4000.
- Tyler JM, Hargreaves WR, Branton D. Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc Natl Acad Sci USA 1979;76:5192-6.
- Tsukita S, Hieda Y, Tsukita S. A new 82-kD barbed end-capping protein (radixin) localized in the cell-to-cell adherens junction: purification and characterization. J Cell Biol 1989;108:2369-82.
- Bretscher A, Chambers D, Nguyen R, et al. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol 2000;16:113-43.
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 2002;3:586-99.
- Pearson MA, Reczek D, Bretscher A, et al. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 2000;101:259-70.
- Chishti AH, Kim AC, Marfatia SM, et al. The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane. Trends Biochem Sci 1998;23:281-2.
- Pearson MA, Reczek D, Bretscher A, et al. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 2000;101:259-70.
- Weinman EJ, Steplock D, Wade JB, et al. Ezrin binding domain-deficient NHERF attenuates cAMP-mediated inhibition of Na(+)/H(+) exchange in OK cells. Am J Physiol Renal Physiol 2001;281:F374-80.
- Darmellah A, Rücker-Martin C, Feuvray D. ERM proteins mediate the effects of Na+/H+ exchanger (NHE1) activation in cardiac myocytes. Cardiovasc Res 2009;81:294-300.
- Martin TA, Harrison G, Mansel RE, et al. The role of the CD44/ezrin complex in cancer metastasis. Crit Rev Oncol Hematol 2003;46:165-86.
- Yonemura S, Hirao M, Doi Y, et al. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J Cell Biol 1998;140:885-95.
- Killock DJ, Parsons M, Zarrouk M, et al. In Vitro and in Vivo Characterization of Molecular Interactions between Calmodulin, Ezrin/Radixin/Moesin, and L-selectin. J Biol Chem 2009;284:8833-45.
- Tynninen O, Carpén O, Jääskeläinen J, et al. Ezrin expression in tissue microarray of primary and recurrent gliomas. Neuropathol Appl Neurobiol 2004;30:472-7.
- Pang ST, Fang X, Valdman A, et al. Expression of ezrin in prostatic intraepithelial neoplasia. Urology 2004;63:609-12.
- Song J, Fadiel A, Edusa V, et al. Estradiol-induced ezrin overexpression in ovarian cancer: a new signaling domain for estrogen. Cancer Lett 2005;220:57-65.
- Khanna C, Wan X, Bose S, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med 2004;10:182-6.
- Salas S, Bartoli C, Deville JL, et al. Ezrin and alpha-smooth muscle actin are immunohistochemical prognostic markers in conventional osteosarcomas. Virchows Arch 2007;451:999-1007.
- Ogino W, Takeshima Y, Mori T, et al. High level of ezrin mRNA expression in an osteosarcoma biopsy sample with lung metastasis. J Pediatr Hematol Oncol 2007;29:435-9.
- Zhang XQ, Chen GP, Wu T, et al. Expression and clinical significance of ezrin in non--small-cell lung cancer. Clin Lung Cancer 2012;13:196-204.
- Elliott BE, Qiao H, Louvard D, et al. Co-operative effect of c-Src and ezrin in deregulation of cell-cell contacts and scattering of mammary carcinoma cells. J Cell Biochem 2004;92:16-28.
- Yu Y, Khan J, Khanna C, et al. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med 2004;10:175-81.
- Ilmonen S, Vaheri A, Asko-Seljavaara S, et al. Ezrin in primary cutaneous melanoma. Mod Pathol 2005;18:503-10.
- Moilanen J, Lassus H, Leminen A, et al. Ezrin immunoreactivity in relation to survival in serous ovarian carcinoma patients. Gynecol Oncol 2003;90:273-81.
- Fernando H, Martin TA, Douglas-Jones A, et al. Expression of the ERM family members (ezrin, radixin and moesin) in breast cancer. Exp Therap Med 2010;1:153-60.
- Arslan AA, Silvera D, Arju R, et al. Atypical ezrin localization as a marker of locally advanced breast cancer. Breast Cancer Res Treat 2012;134:981-8.
- Yeh TS, Tseng JH, Liu NJ, et al. Significance of cellular distribution of ezrin in pancreatic cystic neoplasms and ductal adenocarcinoma. Arch Surg 2005;140:1184-90.
- Kobayashi H, Sagara J, Kurita H, et al. Clinical significance of cellular distribution of moesin in patients with oral squamous cell carcinoma. Clin Cancer Res 2004;10:572-80.
- Yu H, Ye L, Mansel RE, et al. Clinical implications of the influence of Ehm2 on the aggressiveness of breast cancer cells through regulation of matrix metalloproteinase-9 expression. Mol Cancer Res 2010;8:1501-12.
- Jiang WG, Hiscox S, Singhrao SK, et al. Induction of tyrosine phosphorylation and translocation of ezrin by hepatocyte growth factor/scatter factor. Biochem Biophys Res Commun 1995;217:1062-9.
- Hiscox S, Jiang WG. Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. J Cell Sci 1999;112:3081-90.
- Jiang WG, Davies G, Martin TA, et al. Targeting matrilysin and its impact on tumor growth in vivo: the potential implications in breast cancer therapy. Clin Cancer Res 2005;11:6012-9.
- Nazarenko IA, Bhatnagar SK, Hohman RJ. A closed tube format for amplification and detection of DNA based on energy transfer. Nucleic Acids Res 1997;25:2516-21.
- Woodman AC, Sugiyama M, Yoshida K, et al. Analysis of anomalous CD44 gene expression in human breast, bladder, and colon cancer and correlation of observed mRNA and protein isoforms. Am J Pathol 1996;149:1519-30.
- Herrlich P, Pals S, Ponta H. CD44 in colon cancer. Eur J Cancer 1995;31A:1110-2.
- Takeuchi K, Yamaguchi A, Urano T, et al. Expression of CD44 variant exons 8-10 in colorectal cancer and its relationship to metastasis. Jpn J Cancer Res 1995;86:292-7.
- Kelly CP, O’Keane JC, Orellana J, et al. Human colon cancer cells express ICAM-1 in vivo and support LFA-1-dependent lymphocyte adhesion in vitro. Am J Physiol 1992;263:G864-70.
- Jörgren F, Nilbert M, Rambech E, et al. Ezrin expression in rectal cancer predicts time to development of local recurrence. Int J Colorectal Dis 2012;27:893-9.
- Patara M, Santos EM, Coudry Rde A, et al. Ezrin expression as a prognostic marker in colorectal adenocarcinoma. Pathol Oncol Res 2011;17:827-33.
- Zhu J, Feng Y, Ke Z, et al. Down-regulation of miR-183 promotes migration and invasion of osteosarcoma by targeting Ezrin. Am J Pathol 2012;180:2440-51.
- Kinoshita T, Nohata N, Fuse M, et al. Tumor suppressive microRNA-133a regulates novel targets: moesin contributes to cancer cell proliferation and invasion in head and neck squamous cell carcinoma. Biochem Biophys Res Commun 2012;418:378-83.
- Carneiro A, Bendahl PO, Åkerman M, et al. Ezrin expression predicts local recurrence and development of metastases in soft tissue sarcomas. J Clin Pathol 2011;64:689-94.
- Yu Y, Zeng P, Xiong J, et al. Epigenetic drugs can stimulate metastasis through enhanced expression of the pro-metastatic Ezrin gene. PLoS One 2010 ;5:e12710.
- Song J, Fadiel A, Edusa V, et al. Estradiol-induced ezrin overexpression in ovarian cancer: a new signaling domain for estrogen. Cancer Lett 2005;220:57-65.
- Meurice N, Wang L, Lipinski CA, et al. Structural conservation in band 4.1, ezrin, radixin, moesin (FERM) domains as a guide to identify inhibitors of the proline-rich tyrosine kinase 2. J Med Chem 2010;53:669-77.


