Optimal lymphadenectomy for gastric cancer: is there a magic number?
Xu and colleagues propose the ambitious tasks of evaluating in gastric cancer patients “the long-term effect of number of examined lymph nodes on the prognosis of patients,” and exploring “the optimal number of lymph nodes for accurate staging in patients with node-negative gastric cancer after D2 dissection” (1). These two distinctly different goals require very dissimilar analytic strategies. To our surprise, they report one number, not two: 16. The question persists—“Is there a magic number of resected lymph nodes that ensures an optimal lymphadenectomy for gastric cancer?”
How was the analysis done?
The authors use the process of evident differences (“best cutoff”) in survival to identify patient groups based on number of lymph nodes resected. Simply, each group had a range of survival that fell within the confidence limits (Figure 1). This produced 4 groups (1 to 6 nodes resected, 7 to 10, 11 to 15, and ≥16) that did not include an identical range of lymph nodes resected and ignored the quasi-continuous nature of this ordinal variable. The composition of these groups was very different. Patients with ≥16 lymph nodes resected were younger, had more distal gastric cancers, and more T1 and T2 cancers. The authors correctly comment that these factors “influenced the number of nodes resected,” but did not perform any adjustments, thus ignoring these differences and relying solely on univariable analysis.
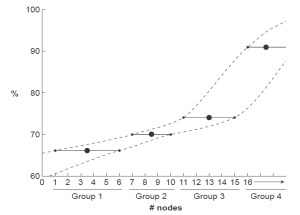
The outcome was disease-specific mortality, a ratio with the numerator being number of deaths attributed to the disease during a specific time interval, and the denominator the size of the population at the midpoint of the interval (2). We are not given details of how the authors actually calculated this outcome. Five-year gastric cancer-specific survival was 66%, 70%, 79%, and 91% for groups 1 to 4, respectively. The authors chose to test the effect of number of nodes resected, dichotomized as <16 and ≥16, on gastric cancer-specific survival in a stepwise univariable fashion with increasing T classification. Survival was similar for the two groups for T1 cancers, but different for T2, T3, and T4 cancers. Although the authors attribute these results to number of lymph nodes resected, these differences may also be explained by difference in group composition. The analysis was not constructed or conducted to identify an exact number; it can only address the unequally dichotomized groups, one with a range of 0 to 15 lymph nodes and the other with an unlimited range of ≥16.
The population studied included only patients free of regional lymph node metastases; this exclusion makes the authors’ second goal of accurate staging unattainable.
What is known?
Recent papers using study groups of variable size and composition and multiple analytic techniques have tried to determine the number of resected lymph nodes that predicts improved survival in patients undergoing gastrectomy for cancer. Huang and colleagues studied 211 node-negative gastric cancer patients and found that to improve survival, ≥15 nodes should be resected for pT1 and pT2 patients and ≥20 nodes for pT3 and pT4 patients (3). Smith and colleagues used SEER data and found a near linear trend between superior survival and number of lymph nodes examined (4). A cut-point analysis revealed the greatest survival difference at 10 lymph nodes examined, but survival improved up to 40 lymph nodes examined. Giuliani and colleagues reported no deaths in node-negative patients with ≥23 lymph nodes resected (5), and Volpe and colleagues reported improved survival in patients undergoing a D2 resection with ≥15 lymph nodes resected (6). None of these articles addresses the number of lymph nodes that need to be resected to produce accurate staging.
The esophageal cancer experience has addressed the authors’ two goals. The number of lymph nodes resected that maximizes overall survival was related to T classification: 10 lymph nodes for pT1, 20 for pT2, and ≥30 for pT3 (7). The number of lymph nodes resected for accurate staging that adequately predicts positive lymph node classification (pN+) is a range that depends on the degree of certainty required. Although the sensitivity of classifying pN+ continued to improve up to 100 nodes examined, maximum increase of sensitivity occurred from 0 to 6 nodes, and over 90% sensitivity was reached at 12 (8). For esophageal cancer, the magic number—the number that maximizes overall survival—is the larger of these two. However, this is not a single number, but one that is dependent on T classification.
What should be done?
It is evident that a single number does not define optimal lymphadenectomy for gastric cancer. Xu and colleagues in their stated purposes outline the dual duties of the surgeon during lymphadenectomy for cancer. A sufficient number of lymph nodes must be excised to accurately stage cancer and to maximize survival. We predict that for gastric cancer, similar to esophageal cancer, it is likely the number of lymph nodes that maximizes overall survival. However, this will not be a single number but will vary depending on other cancer characteristics.
The surgeon should remove as many regional lymph nodes as is safely possible. More is better. There is no magic number.
Acknowledgements
Disclosure: This editorial has not been published or submitted elsewhere. Neither author has a relationship with industry to disclose.
References
- Xu D, Huang Y, Geng Q, et al. Effect of lymph node number on survival of patients with lymph node-negative gastric cancer according to the 7th edition UICC TNM system. PLoS One 2012;7:e38681.
- Available online: www.medical-dictionary.th freedictionary.com
- Huang CM, Lin JX, Zheng CH, et al. Prognostic impact of dissected lymph node count on patients with node-negative gastric cancer. World J Gastroenterol 2009;15:3926-30. [PubMed]
- Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol 2005;23:7114-24. [PubMed]
- Giuliani A, Caporale A, Corona M, et al. Lymphadenectomy in gastric cancer: influence on prognosis of lymph node count. J Exp Clin Cancer Res 2004;23:215-24. [PubMed]
- Volpe CM, Driscoll DL, Douglass HO Jr. Outcome of patients with proximal gastric cancer depends on extent of resection and number of resected lymph nodes. Ann Surg Oncol 2000;7:139-44. [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [PubMed]
- Dutkowski P, Hommel G, Böttger T, et al. How many lymph nodes are needed for an accurate pN classification in esophageal cancer? Evidence for a new threshold value. Hepatogastroenterology 2002;49:176-80. [PubMed]


