|
Cite this article as: Martin TA, Toms AM, Davies LM, Cheng S, Jiang WG.
The clinical and biological implications of N-WASP expression in human
colorectal cancer. Transl Gastrointest Cancer 2012;1:10-20. DOI: 10.3978/
j.issn. 2224-4778.2011.10.01
Original Article
The clinical and biological implications of N-WASP expression in human colorectal cancer
Metastasis & Angiogenesis Research Group, Cardiff University School of Medicine, Cardiff CF14 4XN, UK
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract
Backgrounds: Neural Wiskott-Aldrich Syndrome protein, N-WASP, a member of the WASP family proteins is a regulator of ARP2/3 and cytoskeleton in the cells and has been implicated in regulating cell motility and morphology. N-WASP has been implicated in the development and progression of certain solid tumours. In the present study, we initially investigated the expression levels of N-WASP in a cohort of human colorectal cancers and explored the relationship between N-WASP and clinical outcome. We further examined the impact of N-WASP on the biological functions of colon cancer cells. Materials and methods: A cohort of fresh frozen human colon tissues were used. N-WASP protein in tissues was analysed using an immunohistochemical method. N-WASP transcripts in the tissues were quantified using real-time quantitative PCR methods and correlated with clinical and pathological information of the patients together with clinical outcome. Human colon cancer cell line, HRT18, weakly positive for N-WASP was genetically modified to either over-express N-WASP or to lose N-WASP expression by way of ribozyme transgenes. Cell functions were determined after the genetic manipulation.
Conclusions: N-WASP expression is aberrant in human colon cancer. A reduction of N-WASP in colon tumours is associated with disease progression and a poor clinical outcome of the patients. In addition, N-WASP expression in colon cancer cells is inversely correlated with the aggressiveness of the cells, namely adhesion and invasiveness. Collectively, this study indicates that N-WASP carries the hallmark of a tumour metastasis suppressor in human colon cancer. This is likely to be via a FAK mediated pathway. Key words N-WASP; colon cancer; survival; prognosis; metastasis; cell migration; FAK
Transl Gastrointest Cancer 2012;1:10-20. DOI: 10.3978/j.issn.2224-4778.2011.10.01
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Introduction
Colorectal cancer is one of the most commonly seen cancer throughout the world and is one of the most prevalent tumours in Western countries, including the United Kingdom. In the UK, colorectal cancer is the second and third most common cancers counting for 12% and 13% in females and males, respectively (1,2). Perhaps the most life threatening event in colorectal cancer is the regional and systemic spread of cancer cells, subsequently forming metastatic lesions. Apart from the
traditionally routes of metastasis, namely lymphatic and systemic
spread, colon cancer cells frequently have peritoneal spreading
after penetrating the muscular layers and serious membrane.
Together, locally and systemically advanced diseases are seen in
approximately 20% of the patients. Spreading of cancer cells in
the body, metastasis, is controlled by a number highly relevant
but separate steps collectively known as metastatic cascade,
which itself is influenced by a number of intrinsic and extrinsic
factors in the body and in cancer cells. Factors that are primarily
affecting the metastatic process of cancer cells, without influence
other functions such as cell growth are generally referred to as
metastatic regulating factors including metastatic suppressors
(3,4). Classical metastatic suppressors including nm23, BRMS1
and recently, N-WASP, Neural Wiskott-Aldrich Syndrome
protein has been suggested to be a potential metastatic
suppressor (5-7).
N-WASP is also known as WASL (Wiskott-Aldrich Syndrome
Gene-Like) protein belongs to the WAS family (8,9). Other
members of the WASP family include Wiskott-Aldrich Syndrome
protein family, member-1, also known as WASF-1 or WAVE-1 (WASP family, Verprolin Homology Domain-Containing
protein-1), WASP-2 (or WAVE-2), and WASP-3 (or WAVE-3).
Wiskott-Aldrich syndrome (WAS) or also described as Werlhof ’s
disease was originally described in American kindred where
it was manifested as eczema, thrombocytopenia, proneness
to infection, and bloody diarrhea (10). Death of patients
with WAS were mainly due to infections or bleeding, but also
development of malignancies: lymphoreticular tumors, leukemia
reticuloendothelial system malignancies (11). The N-WASP
protein is a regulator of actin polymerization by stimulating
the actin-nucleating activity of the actin-related protein 2/3
(Arp2/3) complex (12). It has also been shown that the WAS
protein functions as a signal transduction adaptor downstream
of Cdc42 (13). N-WASP has several functional motifs, such as
a pleckstrin homology (PH) domain and cofilin-homologous
region, through which N-WASP depolymerizes actin filaments.
N-WASP-stimulated actin assembly is responsible for membrane
ruffling (14). N-WASP activity is regulated by an intramolecular
interaction that is alleviated following concomitant binding
of Cdc42-GTP to the Cdc42/Rac interactive binding (CRIB)
domain and PtdIns (4,5) P2 to the polybasic region (15).
The N-WASP gene encodes a protein which has 505-amino
acids. WASP is a key regulator of actin polymerization in
hematopoietic cells with 5 domains involved in signalling,
cell motility/migration, in immune synapse formation and in
facilitating the nuclear translocation of nuclear factor kappaB
(13). Mutations of WASP are located throughout the gene and either inhibit or dysregulate normal WASP function: classic
WAS occurs when WASP is absent, X-linked thrombocytopenia
when mutated WASP is expressed, and X-linked neutropenia
when missense mutations occur in the Cdc42-binding site (13).
Despite the fact that N-WASP has been widely studied in cells
including some cancer cells, investigations into the clinical aspect
of N-WASP in human cancer are somewhat hard to come by. In
human breast cancer, we have shown that N-WASP expression
was significantly reduced when compared with normal tissues
and this reduction was associated with poor clinical outcome and
disease progression of the patients (16). In human oesophageal
cancer, there appears to be no significant difference between
tumour tissues and adjacent normal tissues (17). There have
been no reports on studies of N-WASP into human colon cancer.
Here, we report the expression pattern of N-WASP in human
cancer and the biological impact of N-WASP on human colon
cancer cells.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Materials ahd methods
Tissues, cells and materials
Fresh tissues were collected immediately after surgery. Normal
tissues from the same patients were collected from the end of
resected bowels and free from cancer cells. The tissues were
stored at -80oC until use. Patients were followed up in clinics.
Pathological reports and clinical outcomes were recorded and are
shown in Table 1. All procedures were approved by local ethics
committee. Human colon cancer cell line, HRT18 was obtained
from ECACC (European Collection of Animal Cell Cultures,
Salisbury, England, UK). FAK inhibitor, PF573228 was from
Tocris (Bristol, UK). All other materials were from Sigma unless
stated otherwise.
Tissue processing and extraction of RNA and generation of cDNA
Fresh frozen tissues were sections using a cryostat (Leica) at
8μM thickness. A portion of the sections were immediately fixed
and used for histology and immunohistological analysis. The
remaining portions were combined and used for extraction of
total RNA, after homogenised with a hand held homogenizer.
Detection of N-WASP using RT-PCR
Routine RT-PCR was carried out using a PCR master mix that
was commercially available (AbGene). Primers were designed
using the Beacon Designer software (version 2, California,
USA), to amplify regions of human N-WASP that have no significant overlap with other known sequences and that the
amplified products span over at least one intron. The primers
used to amplify N-WASP were given in Table 2. Reactions were
carried out at the following conditions: 94oC for 5 minutes, 36
cycles of 94oC for 15 seconds, 55oC for 25 seconds and 72oC
for 15 seconds. PCR products were separated on a 2% agarose
gel and photographed using a digital camera mounted over a UV
transluminator.
Quantitative analysis of N-WASP
The levels of N-WASP transcripts in cDNA samples from
tissues and cells was determined using a real-time quantitative
PCR, based on the AmplifluorTM technology, modified from
previous reported (18,19). PCR primers are given in table-2
The reaction was carried out using the following: Hot-start
Q-master mix (Abgene), 10pmol of specific forward primer,
1pmol reverse primer which has the Z sequence, 10pmol of
FAM-tagged probe (Intergen Inc), and cDNA from approximate
50ng RNA. GAPDH and CK19 were used as a house keeping
gene and similarly analysed. The reaction was carried out using IcyclerIQtm (Bio-Rad) which equiped with an optic unit that
allows real time detection of 96 reactions, using the following
condition: 94 oC for 12 minutes, 50 cycles of 94 oC for 15
seconds, 55 oC for 40 seconds and 72 oC for 20 seconds. The
levels of the transcripts were generated from a standard that was
simultaneously amplified with the samples and normalised to
CK19 and GAPDH. They are shown here as the N-WASP/CK19
ratio.
Immunohistochemical staining of N-WASP protein
This procedure was similar to a method previously reported
(16). Frozen sections of normal and tumour tissues of colon
were mounted on super frost plus microscope slides and fixed
in a mixture of 50% Acetone and 50% methanol. The sections
were then placed in “Optimax” wash buffer for 5–10 minutes to
rehydrate. Sections were incubated for 20 min in a horse serum
containing blocking solution and probed with the primary
antibody (anti-human N-WASP, Santa Cruz Biotechnologies
Inc., Santa Cruz, California, USA). Following extensive
washings, sections were incubated for 30 min in the secondary biotinylated antibody (Multilink Swine anti- goat/mouse/rabbit
immunoglobulin, Dako Inc.). Following washings, Avidin Biotin
Complex (Vector Laboratories) was then applied to the sections
followed by extensive washings. Diamino benzidine chromogen
(Vector Labs) was then added to the sections which were
incubated in the dark for 5 mins. Sections were then counter
stained in Gill’s Haematoxylin and dehydrated in ascending
grades of methanol before clearing in xylene and mounting
under a cover slip.
Expression constructs for human N-WASP and anti-N-WASP
transgenes
Full length human N-WASP was amplified from normal human
mammary cDNA library as we previously reported using
expression specific primers shown in Table 2 (Martin et al. 2004).
The product was T-A cloned into a pEF6/V5/His-TOPO-TA
(Invitrogen) vector. Anti-N-WASP ribozymes were designed
based on the predicted secondary structure of human N-WASP
(Figure 1A) using short oligos given in Table 2. Ribozymes were
similarly cloned into the pEF6/V5/His-TOPO-TA vectors.
Plasmids with corrected inserted full length N-WASP was
electroporated into human colon cancer cell, HRT18 cells.
Expression of the gene was confirmed by RT-PCR.
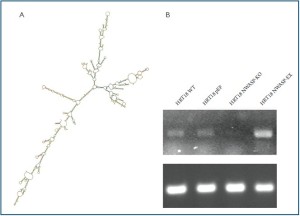
Figure 1. Genetically manipulation of N-WASP expression in human colon cancer cell line. A: The predicted secondary structure of human N-WASP; B: Manipulation of N-WASP expression in HRT-18 cells. HRT18
was weakly positive for N-WASP. Anti-N-WASP transgene successfully knocked down N-WASP expression in
the cell and N-WASP expression construct markedly increased level of expression of N-WASP as revealed by RT-PCT.
In vitro assays analyzing N-WASP gene transformed breast cancer cells
In vitro invasiness. Invasiveness of HRT18 cells were assessed
using the following in vitro assay as previously reported (20). Transwell chambers equipped with 6.5 mm diameter
polycarbonate filter (pore size 8 μm) (Becton Dickinson
Labware, Oxford, UK) were pre-coated with 50 μg/membrane
of solubilised basement membrane in the form of Matrigel
(Collaborative Research Products, Bedford, MA). After
membrane re-hydration, 15,000 cells were aliquoted into each
insert with/without HGF/SF (25 ng/mL). After 96 h co-culture
non-invasive cells were removed with cotton swabs. Invaded
cells on the underside of the insert were fixed and stained with
crystal violet, followed by microscopic counting (20 fields/insert).
Cell growth assay (20). Cells were plated into 96-wells at
3,000 per well. This allowed for 72 hours, after which cells
were fixed with 4% formalin before stained with crystal violet
(0.5% w/w). The rate of cells growth was calculated using the
absorbance of colour staining.
Cytocarrier based cell motility assay (21,22). A cell motility
assay was carried out. Briefly, cells were pre-coated onto
cytodex-2 carrier beads (Sigma-Aldrich, Poole, UK) for 2 hours
in complete medium. After the medium was aspirated and the
cells washed (X2 in complete medium), they were aliquoted into
wells of a 96-well plate in triplicate (300 μl/well). HGF (25 ng/
mL) was added and the cells incubated over-night. The beads
were washed off in medium, and the cells that had migrated onto
the floor of the well fixed (4% formaldehyde) and stained with
crystal violet. The cells were counted microscopically (X40).
Cell-matrix adhesion assay. The cell-matrix attachment assay
was carried out as previously reported23. Briefly, Matrigel (1
mg/well) was added to a 96-well plates, which were incubated
for 24 hours to allow binding of matrix protein to the surface of the well. The plates were then washed and blocked with 5%
BSA (bovine serum albumin). Cells were added at 104/well for
30 minutes, followed by aspiration and washing. The number
of attached cells was determined by direct counting under
microscope.
Statistical analysis
Statistical analysis was performed by MINITAB version 13.32
(Minitab Inc. State College, PA, USA) using a two-sample
student t-test and the non-parametric Mann-Whitney confidence
interval and test, where appropriate. Statistical analysis was
carried out using Mann-Whitney U test and the Kruskal-Wallis
test for tissue samples. Patients long term survival was analysed
using Kaplan-Meier methods with SPSS (version SPAW18)
package.
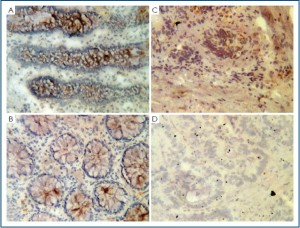
Colon epithelial cells expressed N-WASP, which was reduced in cancer cells
Normal colon epithelial cells stained strongly for the N-WASP
protein. This is primarily seen in the cytoplasmic region of the
cells (Figure 2A, B). Stromal cells are virtually negative for
staining. In colon tumour tissues, however, N-WASP protein
staining was almost negative (Figure 2C, D).
Figure 2. Immunohistochemical analysis of human N-WASP in human colon tissues. A and B: normal
colon tissues; C and D: colon tumour tissues.
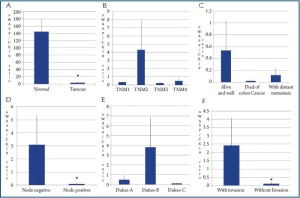
Levels of N-WASP transcripts and the clinical and pathological links
Perhaps the most striking observation with levels of N-WASP
in patients clinical samples is the significantly lower levels
of N-WASP in tumour tissues compared with normal tissue
(P<0.0001) (Figure 3A). Both node positive tumours and
tumour with muscular invasion had significantly lower levels of N-WASP compared with node negative and non-invasive
tumours (Figure 3D, F, respectively). When tumour staging
is compared, there does not appear to be a clear link between
N-WASP and TNM/Dukes staging. For example, TNM-2
tumours had higher levels of N-WASP than TNM1 and Dukes-B
higher than Dukes-A tumours (Figure 3B, E). It is nonetheless
interesting to observe that the most aggressive stages of the
tumours, namely TNM3/4 and Dukes-C had lower levels
than the moderate aggressive TNM-2 and Dukes-B tumours,
although statistically this has yet to reach a difference. Finally,
compared with patients who are alive, patients who died of colon
cancer related causes had lower levels of N-WASP, although the
difference is not statistically significant (P=0.068). Similarly,
tumours from patients who developed distant metastasis also
showed levels of N-WASP than tumours from those patients
who remained disease free.
Figure 3. Expression of N-WASP transcript and the associated with clinical and pathological features. A: comparison between normal and tumour tissues; B: N-WASP and TNM staging; C: N-WASP and clinical
outcome; D: N-WASP and nodal status; E: N-WASP and Dukes staging; F: N-WASP and tissue invasion by
tumour cells. Shown are N-WASP/CK19 ratio. *P<0.05.
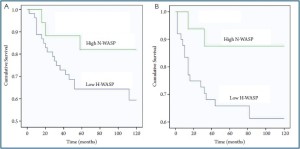
The relationship between N-WASP and long term survival
At the end of followup, patients were divided into those who
were disease free, those who had developed distant metastasis
and those who died of colon rectal cancer (Table 1). Using the
Kaplan-Meier survival model, we analysed the expression pattern
and the long term survival of the patients. As shown in Figure 4A,
patients with low levels of N-WASP had a shorter overall survival
[117 months (94-140) months] compared with those with higher
levels [156 (131-181) months]. Similarly, low levels of N-WASP
also associated with a shorter disease free survival [119 (98-140) months vs. 150 (122-177) months, for low and high levels,
respectively)] (Figure 4B).
Figure 4. N-WASP expression and patients long term survival using Kaplan-Meier survival analysis. A: overall survival; B: disease free survival. Patients with low levels of N-WASP had short overall survival and disease free survival.
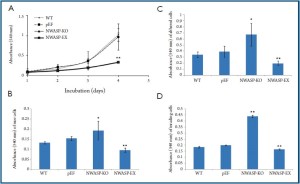
Effect of N-WASP expression on the cellular function of colon cancer cells
Over-expression of N-WASP in HRT18 cells significantly
reduced the rate of cell growth compared with control cells,
although the effect of knocking down was less marked (Figure 5A). Interestingly, knocking down N-WASP significantly
increased the adherence of the cells to extracellular matrix
(Figure 5B). A significant opposite effect was seen when N-WASP was over-expressed. A similar pattern of relationship
between N-WASP and cell motility (Figure 5C) and cell
invasiveness (Figure 5D) was seen.
Figure 5. Expression of N-WASP and the biological function in colon cancer cells. A: Cell growth
assay; B: Cell-matrix adhesion assay; C: cell motility assay and D: cell invasion assay. * and **: Pvs. wild type and control cells.
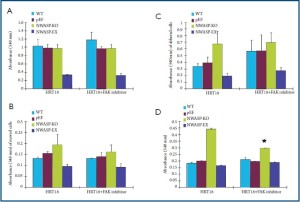
The potential involvement of the FAK pathway in N-WASP mediated cellular functions
In order to explore the potential role of the FAK in N-WASP mediated cellular functions, we exmployed a small specific FAK inhibitor in the cellular functions (Figure 6). The inhibitor had no marked effect on the growth and motility of the cells. However, it significantly reduced te invasiveness due to N-WASP knock down (Figure 6D).
Figure 6. The potential role of FAK pathway in N-WASP mediated cell functions. A: Cell growth
assay; B: Cell-matrix adhesion assay; C: cell motility assay and D: cell invasion assay. * p<0.05 vs.
without FAK inhibitor.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Discussion
The present study has demonstrated that N-WASP, a potential
cancer progression ? associated protein, had an aberrant
expression pattern in human colon cancer. The study has shown
that colon cancers had markedly lower levels of N-WASP
at gene transcript and protein levels, as shown by both
immunohistochemistry and quantitative PCR. The study further
demonstrated that the levels of expression were associated with
nodal status and muscular invasion and a low level was associated
with a poor clinical outcome of the patients. This link appears to
be reflected by the biological impact of N-WASP on colorectal
cancer cells, in that levels of N-WASP were linked to the growth
and invasiveness, possibly via the FAK pathway.
Colon tissues are highly positive for N-WASP and the
N-WASP protein was largely seen in the cytoplasmic region
of normal colonic epithelial cells. It is also interesting to
observe that N-WASP protein staining was stronger in mature
and differentiated epithelial cells compared with basal cells
(Figure 2A, B). Tumour cells, from tissues and cell line alike,
had much lower levels of N-WASP compared with normal
cells. An interesting feature of the study on clinical samples
is the inverse link between levels of N-WASP, lymph node
involvement and tumour cell invasion of muscular layer. This
inverse relationship is well reflected in the long term followup
and patient’s clinical outcome, namely low levels of N-WASP
were seen in patients who died of colon cancer and the Kaplan-
Meier model confirmed that these low levels were associated
with shorter overall survival and disease free survival. Together,
the data attempts to demonstrate a strong link between N-WASP
and tumour invasiveness and clinical outcome. However, this
link does not appear to be supported by the analysis on tumour
staging (TNM and Dukes stagings). In that early tumours,
namely TNM-1 and Dukes-A appear to have lower levels of N-WASP compared with TNM-2 and Dukes-B tumours. Two
possible reasons may have contributed to this observation,
firstly the relatively smaller number of sample size in TNM-
1 and Dukes-A groups; secondly, N-WASP may not be a good
indicator for tumour staging. Clearly, a larger cohort would help
to resolve this matter.
N-WASP belongs to a larger protein family, which include
WASP, WAVEs and WISPs. Expression of N-WASP and the
clinical implications of N-WASP have been reported in other
tumour types. For example, in human breast cancer, N-WASP
was reported to be expressed in a pattern similar to that reported
here, namely reduced level of expression in aggressive tumours
(16). In addition, other WASP family members have been
studied in human colon cancer. WISP family has a different
expression pattern from N-WASP. Aggressive colon tumours
have markedly raised levels of WISP-1, whereas levels WISP-
2 barely changed (24). An opposite trend was seen with breast
cancer, in which WISP-1 was reduced in aggressive breast cancer
cells and WISP-2 increased in the same tumours (25). Apart
from the WASPs, the other members of the family have also
been shown aberrant in human cancer. For example, WAVE-3 has been shown to connected to the disease progression
and aggressive behaviour of prostate cancer and breast cancer
(26,27). Again in human colorectal cancer, WAVE-2 has been
indicated in the disease progression and clinical outcome of the
patients (28).
To further understand the biological role of N-WASP in the
behaviour of colon cancer cells, we created sublines from human
colon cancer cell line, HRT-18, a cell weakly positive for N-WASP
expression. Here, we created a subline in which N-WASP
expression was knocked out and a subline N-WASP overexpressed.
It was clearly demonstrated that over-expression of
N-WASP markedly decreased the growth, adhesion and in vitro
invasiveness of the cancer cells, with opposite effect seen when
N-WASP was knocked down. The present study further revealed
that inhibition of FAK by way of FAK inhibitor blocked increase
in invasion due to N-WASP knockdown. It has shown previously
that the FAK is able to affect the cellular location and activation
of N-WASP in the cells thus the matrix adhesion of cells (29).
It has also been reported that in breast cancer cells oestrogen is
able to induce activation of FAK and the interaction between
FAK and the N-WASP/ARP2/3 complex, which regulates cell
migration (30). The present study is unable to provide further
mechanism by which FAK and N-WASP interplay in colon
cancer cells, but these recent studies provides vital information
on the link between the two cellular protein complexes and will
be useful future leads to explore, including mechanism beyond
the FAK pathway (31).
In conclusion, N-WASP expression is aberrant in human
colon cancer. A reduction of N-WASP in colon tumours is
associated with disease progression and poor clinical outcome
of the patients. In addition, N-WASP expression in colon
cancer cells is inversely correlated with the aggressiveness of
the cells, namely adhesion and invasiveness. Collectively, this
study indicates that N-WASP carries the hallmark of a tumour
metastasis suppressor in human colon cancer. This is likely to be
via a FAK mediated pathway.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Acknowledgements
The authors wish to thank Cancer Research Wales and the Albert
Hung Foundation for supporting their work.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||