Cholangiocarcinoma: Epidemiology and risk factors
Abstract
Cholangiocarcinoma (CCA) is a malignant tumour, arising from biliary epithelium at any portion of the biliary tree, characterized by a bad prognosis and poor response to current therapies. CCA is currently classified as intrahepatic (IH-CCA) or extrahepatic (EH-CCA). The distinction between IH-CCA and EH-CCA has become increasingly important, as the epidemiological features (i.e., incidence and risk factors), the biologic and pathologic characteristics and the clinical course are largely different. New insights into hepatic and biliary tree stem cell niches organization, into cancer cells of origin and cancer stem cell biology are currently under evaluation as the biological bases of the observed heterogeneity of CCA and could explain the differences in epidemiology and risk factors between IH- and EH-CCA. The purpose of this manuscript is to revise recent literature dealing with the descriptive epidemiology, risk factors and clinical-pathological heterogeneity of CCA with a special effort to compare IH- versus EH-CCA.
Key words
Intra-hepatic cholangiocarcinoma; Extra-hepatic cholangiocarcinoma; Epidemiology; Risk factors; Incidence
Introduction
Cholangiocarcinoma (CCA) arises from the malignant proliferation of cholangiocytes, the epithelial cells lining the biliary tree, and is characterized by a bad prognosis and poor response to current therapies. CCA may emerge at any portion of the biliary tree and includes a group of tumors largely heterogeneous from epidemiologic, morphologic, biologic and clinical point of view. CCA is currently classified as intrahepatic (IH-CCA) or extrahepatic (EH-CCA), the second-order bile ducts acting as the separation point (1,2). The EH-CCA is comprised of the perihilar form (Klatskin tumor) and distal form where the separation point being posed at the level of the cystic duct. The distinction between IH- and EH-CCA has become increasingly important, as the epidemiological features (i.e., incidence and risk factors), the biologic and pathologic characteristics and the clinical course are largely different (1,2).
Epidemiology
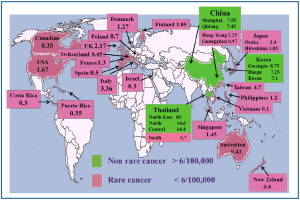
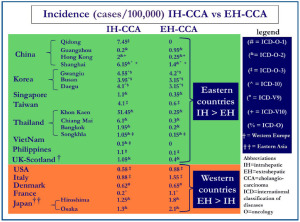
The epidemiologic data on CCA and its different forms are affected by the lack of worldwide uniform classification (1-5). In general, a number of biases and criticisms should be taken into consideration in evaluating the literature dealing with epidemiology and risk factors of CCA and especially of the two main forms, the IH- and EH-CCA: (I) in many cancer registries CCA is combined with other primitive liver cancers and the perihilar EH-CCA has been often considered as IH-CCA. Indeed, some authors described the epidemiology and the risk factors for CCA clearly considering the Klatskin TOMOR (8162) as IH-CCA; (II) until 2006, perihilar CCA was assigned by ICD-O (International Classification of Diseases Oncology)-2 to unique histology code (8162/3, Klatksin), rather than a topography code. In contrast, the ICD-O-3 cross-referenced perihilar CCA to topography codes for either IH-CCA or EH-CCA. This has been estimated by Welzel et al. (1,2) to result, before application of ICD-O-3, in over reporting of IH-CCA by 13% and underreporting of EH-CCA by 15 %; (III) in most cancer registries, at least 30% of CCA are classified as NOS (not otherwise specified localization); (IV) as indicated by the European RARECARE (7) project, more recent studies identified IH-CCA by specific topographical (C22.0, C22.1) and morphological codes (8000–8005, 8010, 8011, 8020–8022, 8050–8084, 8140–8141, 8160, 8161, 8480–8500, 8550, 8560, 8570–8572) while EH-CCA has been identified by the topographical codes C24.0 and the morphological codes 8000–8005, 8010, 8011, 8020–8022, 8140–8141, 8144, 8160–8161, 8162 (Klatskin tumour), 8190, 8230–8231, 8260, 8310, 8480–8500, 8550, 8560, 8570–8573, 8575–8576. CCA with not otherwise specified localization is finally identified by topography code 24.9, irrespective of histology. Current efforts to characterize and define the cell of origin of CCA and other primitive liver cancers could help in the near future; (V) diagnosis is frequently performed in advanced stage when distinction between IH- and EH-CCA is challenging; (VI) the histological heterogeneity of CCA and the lack of specific immunohistochemical markers favor frequent misclassification (1-5). Indeed, pathological examination is generally considered the “gold standard” for the diagnosis, and consequently, a high degree of confidence is placed in microscopically verified cancer registrations (8). Unfortunately, as described in an Italian series of incident CCA, even in tertiary centres, tissue-proven diagnosis was obtained in less than 80% of the cases (9). Further, (VII) mixed HCC (hepatocarcinoma)-CCAs are often considered as IH-CCA. In a population of clinically diagnosed IH-CCA, for example, the proportion of HCC-CCA has been reported to be 30%, as definitively demonstrated in surgically resected pieces (10). At moment, it is impossible to define whether biological and clinical behavior are similar between classical IH-CCA, mixed HCC-CCA and cholangiolocellular CCA (CCL). With all these considerations in mind, the incidence of CCA shows large geographic variation (1-5). The description of the incidence of the CCA in the areas depicted in Figure 1, takes in consideration cases diagnosed either clinically (ICD-9, -10, others classifications) or cases verified by morphology (ICD-O-1, ICD-O-2, ICD-O-3). Very high incidence rates (85/100,000) has been reported in northeast Thailand, where CCA represents approx. 85% of total primitive liver cancers (11) and other Asian countries such as China and Korea (12). In particular, in Thailand, large variation in the incidence exists among different regions. While in northeast Thailand (Khon Kaen province) the incidence rates were 85/100,000, in north, central and south of this country, the incidence rates were 14.6/100,000, 14.4/100,000 and 5.7/100,000 respectively (13). The variation in incidence rates in the Thailand regions correlated with the different prevalence of liver flukes. In China also the incidence data are heterogeneous. Data from the population-based cancer registry in Shanghai, indicate that CCA has increased more rapidly than other malignancy (14). From 1998 to 2002 the CCA incidence in Shangai and in the Quidong region was 7.55/100,000 and 7.45/100,000 respectively, while in Guangzhou and Hong Kong it was 0.97/100,000 and 2.25/100,000 respectively, in the same period (12). The reasons for the rising incidence of CCA in Shangai and Quidong are unclear although improvement in diagnosis and classification may have contributed to the trend. Korea is another country with high incidence of CCA; in the period 2003-2005 it was > 6/100,000 (Gwangiu 8.75/100,000, Busan 7.1/100,000 and Daegu 7.25/100,000). In this country, from 1999 to 2005 IH-CCA incidence rates increased gradually while were stable for EH-CCA. The reasons for the selective increase in IH-CCA incidence are not clear. Part of this increase may be attributed to changes in the coding system, from ICD-O-2 to ICD-O-3 (15), but, this is not the unique reason since incidence rates of IH-CCA continue to raise even if incidence of primitive liver cancers is decreasing. The Taiwan and Japanese population have relatively higher rates of CCA incidence (Hiroshima 3.05, Osaka 3.4, Taiwan 4.7/100,000) than others Asian populations such as Singapore, Philippines and Viet Nam (1.45/100,000, 1.2/100,000 and 0.15/100,000 respectively) (15,16). In comparison with the eastern countries, lower incidence rates occur in all western countries. The age-standardized incidence in New-Zeland (0.4/100,000), Costa Rica (0.3/100,00), Puerto Rico (0.35/100,000), Israel (0.35/100,000), Australia (0.3/100,000) and Canada (0.43/100,000) was estimated to be about a third the world-wide incidence (17,18). Particularly explicative is Australia, where residents born in other countries and especially migrants from Asia, have a higher incidence of CCA than indigenous. However over the last years, even in Australian an increased CCA incidence has been registered since in the years 1977-1982 CCA represented 1.6 % of total primitive liver cancer while, in the period 2003-2007, incidence of CCA was 19.22% of the total (17). Also in Europe CCA is a rare cancer (<6/100,000) with very low rates registered in Spain, Poland and Switzerland (0.5/100,000, 0.7/100,000 and 0.45/100,000 cases respectively) (18). In addition, large regional differences in the temporal trend of IH-CCA and EH-CCA incidence have been registered in Europe. The incidence of both IH-CCA and EH-CCA is decreasing in Denmark, stable in France and increasing in Italy and Germany where the increase mainly affect the IH-CCA. In Denmark, the CCA incidence decreased from 2.20/100,000 (1978-1982 period) to 1.27/100,000 (1998-2002 period) (19), however, Montomoli et al. observed how, CCA represented 13.8% of total liver cancer in the period 1998-2000 and 18.4% in the period 2007-2009 (20). The data from the United Kingdom (21) showed that in the period 1971-1973, IH-CCA was rare in both males and females but over the subsequent three decades the incidence rate increased by 12 times while, in contrast, the incidence of EH-CCA decreased markedly (22). In Italy, in the last decade (1995-2005), CCA incidence was 3.36/100,000, where CCAs accounted approximately for 3% of total primitive liver cancers. An important linear increase in CCA incidence has been registered in the last two decades in Italy, crude incidence rates being higher for EH- than for IH-CCA, but the average percentage of increase per year was higher for IH-CCA (5). In the decades 1979-1999, in many western countries and some eastern countries, a progressive increase of IH-CCA incidence has been reported, while the incidence of EH-CCA was stable or slightly decreasing (12,14). Specifically, this trend have been reported for Italy, Germany, Scotland/Wales, USA, Australia, Japan and Korea. More recent data on incidence showed that in USA incidence rates of IH-CCA decreased from 0.85 (1995-1999) to 0.58 (2000-2005), is continuing to decrease in Denmark, to be stable in France and Germany and is continuing to increase (but less than previous years) in Italy and Korea. In substance, although further confirmation is need, the trend of IH-CCA incidence in the last 3-4 decades looks similar to that of HCC but with a delayed plateau. Currently, on the basis of the incidence data, IH-CCA represents the vast majority of primitive liver cancers in some countries (i. e. Thailand, Kon Khan=85%), while it accounts for 10-17% in Korea (14%), USA (10.7%) and Australia (16.5%), and only 3-5 % in Italy and Japan. As far as EH-CCA is concerned, incidence is stable or slightly decreasing in the majority of countries with the exception of Italy and Germany. While in the early epidemiological reports this was in part due to the combination of EH-CCA with gallbladder cancer, more recent data, considering EH-CCA separately, have confirmed the trend. In general, incidence of EH-CCA is higher than that of IH-CCA in western countries and Japan while, the opposite occurs in eastern countries (Figure 2). In all reports, the incidence of IH-CCA and of EH-CCA is higher in men than women.
CCA clinical-pathological heterogeneity: A major bias in epidemiological investigation
Recent studies further stressed the concept of CCA heterogeneity. Nakanuma et al. recently proposed a new classification of CCA taking into consideration human hepatic stem cells niches and the pathological similarities between biliary and pancreatic neoplasms (23). The authors classified IH-CCA into: (I) bile ductular type or CLC; (II) intraductal neoplasm type; (III) conventional (bile duct) type and; (IV) rare variants (23). CLC is thought to originate from canals of Hering/bile ductules where human hepatic stem/progenitor cells (hHpSCs) are located. Komuta et al. showed that this subtype of CCA is mainly composed of CLC areas showing small monotonous and/or anastomosing glands, strongly positive for keratin (K) 7 and K19, with tumour boundary being characterized by HCC-like trabecular area and with some cases expressing CCA areas with scarce mucin production (24). Comparison of CLC with K19-positive HCC and with combined HCC-CCA indicated a high homology (24). The evidence that IH-CCA and EH-CCA may be dissimilar tumours is supported by the recent discovery that, in vitro, they express different cellular proteins and have different cellular shape, doubling time, chromosome karyotype and chemosensitivity (25). Similarly, researchers from France showed that perihilar CCA express higher levels of MUC5AC (60% vs. 22%), Akt2 (64% vs. 36%), K8 (98% vs. 82%), annexin (56% vs. 44%) and less vascular epithelial growth factor (22% vs. 78%) in comparison to IH-CCA (26). Moreover, prognostic markers resulted differentially expressed, as perihilar CCAs carried out stronger perineural invasion (83% vs. 42%, P<0.001) than peripheral cholangiocarcinomas (26). The different biological and molecular features strongly support the concept that IH-CCA and EH-CCA arise from different carcinogenetic processes and different cells of origin. Particularly relevant in the view of future clinical trials is the lower expression of vascular endothelial growth factor in EH-CCA with respect to the IH-CCA, which could affect the response to anti-angiogenic based therapy. Recently, Roskams et al., carried out a study aimed to investigate the CCA histological diversity in relation with the heterogeneity of cholangiocytes lining the biliary tree: hilar mucin producing cells versus peripheral cuboidal ductular cells or hHpSCs (27). They investigated the clinical-pathological and molecular features of 79 resected CCAs and their relationship with hHpSCs and, compared the spectrum of CCAs with respect to K19-positive or negative HCCs. According to this study, 52% of the CCA were pure mucin producing whereas 48% showed mixed differentiation features including focal hepatocytic differentiation and CCL features. CCA with mixed features (mixed-CCAs) showed peripheral location, larger tumor size, less micro-vascular invasion, less lymph node involvement compared to pure mucin producing CCAs which showed hilar location, smaller tumour size, more microvascular invasion and more lymph node involvement. S100p expression was seen only in CCAs, while neural cell adhesion molecule (NCAM) expression was only present in mixed-CCAs. Molecular profiling showed high homology between mixed-CCAs and K19-positive HCCs (considered of HpSCs origin). The authors concluded that mixed-CCAs and K19-positive HCCs have a similar molecular profile as the most peripheral ductules, containing hHpSCs, while mucin producing CCAs have a similar profile to mucin producing large IH and EH bile ducts, possibly reflecting the different cells of origin (27). On the basis of this recent study, it is obvious that approximately half of IH-CCA cases displays biology and perhaps oncogenesis similar to EH-CCA and this should be taken in adequate consideration in future epidemiologic studies. Differences in clinical-pathological features between CCAs arising from small (interlobular bile ducts) or medium/large IH bile ducts are under investigations. Responding to the need of classifying IH-CCA in relation to the hetereogenity of the small vs. the medium-large IH bile ducts, recently Nakanuma et al. proposed to separately consider a small duct type (peripheral type) and a large bile duct type (perihilar type) (23). The former is mainly described as a tubular or micropapillary adenocarcinoma while the latter involves the IH large bile ducts. In accordance with phenotypical differences between interlobular and medium-large bile ducts, Aishima et al. investigated 87 cases of IH-CCA smaller than 5 cm in diameter (28). They considered a perihilar type IH-CCA, showing IH large bile duct involvement within the tumour, and a peripheral type contained preserved architecture of the portal triad. They demonstrated that the frequency of perineural invasion, lymph node metastasis, vascular invasion, intrahepatic metastasis and extrahepatic recurrence of IH-CCA from large ducts was significantly higher than that of IH-CCA from small ducts (28). The survival of patients with IH-CCA from large ducts was worse than that of patients with IH-CCA from small ducts (28). In our hypothesis the clinical-pathological differences observed among CCAs arising from small bile ducts and large bile ducts reflect the different lineage of origin, with the former arising from cells of the hHpSC lineage and the latter arising from cells of the PBGs derived lineage.
Risk factors
In Table 1 the definite and probable CCA risk factors are reported. It is clearly evident from the literature that there are pathologies exclusively associated with IH-CCA or EH-CCA and pathologies associated with both. Especially hepatic infections by liver flukes (Clonorchis sinensis, Opisthorchis viverrini) are associated with the high incidence of IH- and EH-CCA in Asian countries (3,29,30). However, as recently described (29) all the pathologic conditions primarily affecting large IH bile ducts and/or extra-hepatic bile ducts, as choledochal cysts, cholangitis/primary sclerosing cholangitis (PSC), secondary biliary cirrhosis, choledocholithiasis and liver flukes, resulted associated with both IH- and EH-CCA development. Pancreaticobiliary maljunction (PBM) with bile duct dilatation, cholelithiasis and cholecystectomy are associated exclusively with EH-CCA other than gallbladder cancer. In contrast, HCV, HBV, hepatic schistosomiasis, hepatolithiasis and cirrhosis are associated exclusively with IH-CCA (Table 1). Both hepatic infections by liver flukes (Clonorchis sinensis, Opisthorchis viverrini) and hepatolithiasis are associated with the high incidence of IH-CCA in Asian countries (3,29,30). Pathologies of the large IH and EH bile ducts, such as liver flukes, cholangitis, PSC, choledochal cysts, secondary biliary cirrhosis, choledocholithiasis, cholecystitis are characterized by the involvement of peribiliary glands (PBGs) where, cells proliferate and acquire the expression of stem cells and neuroendocrine markers (C-met, c-erbB-2, argirophil granules, chromogranin A) (31-34). On the basis of these recent observations, we hypothesize that early cells within PBGs are the sites of origin of malignancies associated with chronic diseases or pathologic conditions of the IH medium/large and EH bile ducts (29,35). Without considering affections of the gallbladder (cholecystectomy and cholelithiasias), which are clearly associated specifically whit EH-CCA (Table 1), interestingly only one condition, the pancreaticobiliary maljunction (PBM), has been associated exclusively with EH-CCA development. Indeed, in patients with PBM, a congenital anomaly of the union of the pancreatic and biliary ducts that is located outside the duodenal wall associated sphincter system, EH-CCA was diagnosed in 2 to 27% of the patients according to different case series (mostly Japanese patients) (36,37). This rate of EH-CCA incidence was largely higher than in general population. The incidence of PBM is thought to be 1.5-2.0% from the data on patients receiving ERCP (endoscopic retrograde cholangiopancreatography) for biliary disorders (38). Howerer, by using endoscopic ultrasonography and ERCP, Yamao et al. reported the incidence of PBM to be 0.03% in a prospective study of 27076 subjects, most of whom were asymptomatic and showed normal laboratory data (39). It is believed that PBM occurs more frequently in asiatic patients (38). Controversies exist whether bile duct dilatation confers to PBM additional risk for EH-CCA (36,37). Since the diagnosis of PBM is now performed by MRCP (magnetic resonance cholangiopancreatography), this risk factor should be taken in adequate consideration for surveillance. PBM is a risk factor also for gallbladder cancer and therefore, Japanese scientific societies recommended prophylactic excision of gallbladder and of common bile duct in patients with PBM and bile duct dilatation (37). PBM is frequently associated with bile duct cysts (BDC) of all types of the Todani classification although type I and IV cysts show a higher cancer incidence (39,40). CCA is found in 10–30% of adults with BDC, the risk is low in childhood but tends to increase with age. In general, in patients with PBM or BDC, CCA emerges at younger age than in the absence of these malformations (36,37,39,40). The mechanism of carcinogenesis in PBM is still unknown, the reflux and stasis of pancreatic juice in common bile duct with enhanced biliary concentration of detergents including lysolecithin have been considered possible pathogenic mechanisms. In contrast with pathologies involving both large IH and EH bile ducts such as PSC, parenchymal liver diseases including chronic viral and non-viral liver diseases and schistosomiasis, exclusively target interlobular bile ducts, bile ductules and the canals of Hering. Consistently, these pathologies are exclusively associated with development of only IH-CCA. These pathologies are characterized by a strong ductular reaction, a phenomenon involving interlobular bile ducts, bile ductules and canals of Hering, which is currently considered as the morphological expression of the activation of the stem/progenitor cells compartment aimed to repair liver injury (41). Therefore, parenchymal liver diseases, and especially liver cirrhosis, could be considered as the diseases associated with CCAs arising from the hHpSCs derived lineage (29). CCA is a prototype of inflammation-associated cancer and indeed, all risk factors share, as common feature, chronic inflammation of bile ducts other than cholestasis and activation/proliferation of resident stem cells (29). However, in more than 60% of the cases of CCA no putative risk factor is detectable, indicating that very few is still know on this topic. Very recently, greater attention has been given to the cells of origin of CCA and to the role of the cancer stem cells in sustaining the tumor progression and malignancy (42-45). To this regard an important advance could be indicated by the identification of stem cell niches in PBGs of the biliary tree (35,46). Indeed, PBGs are particularly high in density at the level of cystic duct, hilum and periampular region, sites where EH-CCAs typically emerge (35,46,47). Hepatic stem cell niches, residing in the canals of Hering, and biliary tree stem cell niches, residing in the PBGs, are differently activated in the course of hepatic and biliary pathologies (29,35) . This observation could have important pathological implications in the link between risk factors and CCA emergence (29,35). As previously observed in this report, a biological based classification of the CCAs, taking in count the new insights in liver stem cell niches, could explain either the differences in IH- and EH-CCA incidence among different countries or the different temporal trend. In general, the geographic differences in incidence can be explained by differences in the prevalence of the different risk factors. Endemic areas for liver flukes infection, such as Thailand and China (48), are also the regions with the highest worldwide incidence of both IH- and EH-CCA. In the different regions of Thailand, mortality rates for CCA strongly correlated with the prevalence of liver flukes infections (12). For example, in Northeast Thailand mortality rate for CCA is 43.6/100,000 and Opisthorchis viverrini prevalence is 16%, while, in South Thailand, where the prevalence Opisthorchis viverrini is around zero, mortality rate for CCA is only 4/100,000 (12). Interestingly, although liver flukes are well recognized risk factors both for IH- and EH-CCA, in endemic areas as well as in other eastern countries with low liver flukes prevalence (Korea, Singapore, Taiwan, and Philippines), the incidence of IH-CCA is higher than that EH-CCA; the opposite occurs in western countries where the incidence of EH-CCA largely predominates (Figure 2). A possible explanation for these geographic differences is related to the epidemiology of hepatitis viruses. The global HCV prevalence, according to World Health Organization (WHO), is estimated to be 2%, one third the prevalence of HBV (49). However, HBV and HCV prevalence gradually decreases passing from eastern developing countries trough Japan and Southern Europe, to Western Europe and North America. Indeed, a very high prevalence of viral hepatitis infection occurs in developing countries including South-Eastern Asia (HBV 9.1 %, HCV 3.6), China (12%, 3%), Korea (12%, 2%), intermediate prevalence exists in Japan (2%, 2.3%) and Southern Europe (2.8%, 0.7%), while, Western Europe (0.5%, 0.5%) and North America (0.5%, 1.6%) showed the lowest prevalence of viral hepatitis infection (48,49). Since HCV and HBV have been considered risk factors exclusive for IH-CCA, the geographic differences in the relative incidence of IH-CCA versus EH-CCA well reflect the epidemiology of hepatitis viruses infection. Another example is the reported increased incidence of IH-CCA (but not EH-CCA) in western countries in the decades 1980-2000, which has been related with the parallel burden of HCV, and this has been clearly demonstrated in USA (50). That HCV represents a definitive risk factor only for IH-CCA has been definitively ascertained. Recently, a large cohort study (follow-up 8 years) demonstrated that HCV infection significantly increased the risk of clinically diagnosed HCC (155.0) and IH-CCA (155.1 ICD-9), although the OR was 5 fold higher for HCC than IH-CCA, whereas EH-CCA (156.1, 156.2, 156.8 and 156.9) and pancreatic adenocarcinoma (157.0, 157.1, 157.2, 157.3, 157.8 and 157.9) resulted not associated with HCV infection (51). Yamamoto et al. (52) revealed by multivariate analysis that during chronic hepatis C without cirrhosis the adjusted OR for ICC was 2.32. In a recent metanalysis of 10 published studies, HCV positivity doubled the risk of CCA (OR=1.831); in 7/10 studies only IH-CCA was analyzed but, in the 3 remaining studies where IH- and EH-CCA were considered separately, HCV was significantly associated only with IH-CCA but not EH-CCA (4). In these studies, the association between HCV and CCA has been evaluated independently from the degree of liver damage. However, the trend indicate that more advanced is the liver injury correlated with HCV-infection more enhanced is the risk of IH-CCA and this is consistent with studies indicating how liver cirrhosis (unspecified aetiology) is a CCA risk factor (see above). HBV also is considered a risk factor exclusive for IH-CCA but the evidence are less consistent than for HCV. In a cohort study of asymptomatic subjects, HBsAg-positivity confers a significantly higher risk for IH-CCA (histological and/or clinical diagnosed) and the risk was further increased in HBsAg positive patients with ALT level of 40 KU or higher (53). The OR for HBsAg seropositivity in the positive study, goes from 2.3 to 9.7. In a recent metanalysis of 10 studies, HBsAg positivity was associated with CCA with OR of 4.84; in 7 out of 10 studies only IH-CCA was investigated and in 3 of these studies the association was insignificant while, in 2 out of 3 studies where EH-CCA was also investigated the association was insignificant (L1). In a recent prospective study performed in a cohort of parous women in Taiwan, the incidence of IH-CCA in HBsAg+ versus HBsAg- was 0.43 vs. 0.09/100,000 per year (HR=4.8) (54). In general, higher is the prevalence of HCV or HBV infection in the geographic area investigated, higher the level of significance in the association between hepatitis viruses and CCA. Liver cirrhosis is another risk factor exclusively associated with IH-CCA. In recent studies, at least 20-30% of CCA emerge in the setting of liver cirrhosis and this is a challenge for the differential diagnosis between HCC and IH-CCA based on imaging procedures. Lee et al. (55) demonstrate that cirrhosis increased the risk for IH-CCA by 3.2 fold in HCV patients. Yamamoto et al. (52) revealed by multivariate analysis that as liver status worsened during HCV infection, the adjusted OR for IH-CCA tended to increase (chronic hepatitis, 2.32 vs. cirrhosis, 5.03). As for HCV, the presence of HBV-related cirrhosis increases the OR for IH-CCA development from 7.3 to 18 according to Tao et al. (56), and from 9.7 to 13 according to Zhou et al. (57). Compelling with this evidence Lee et al. (55) demonstrate that cirrhosis increased the risk for IH-CCA by 3.2 fold in HBV patients. Lee et al. (55) described that IH-CCA patients with hepatitis B (56.4+/-11.1 years) are 9 years younger than IH-CCA patients with hepatitis C (65.6+/-9.17 years), similar to that observed for HCC. Taking altogether the current literature, as already clarified for HCC, the association between IH-CCA and hepatitis viruses (HBV, HCV) is markedly stronger when cirrhosis is present, suggesting that the background of enhanced proliferation and cell cycle acceleration, represents, together with chronic inflammation, a significant pathogenic determinant. Hepatic stem cell activation accompanies many forms of Hepatic damage irrespective of aetiology, making such cells very likely carcinogen targets (58). HBV (59) and HCV (60) components have been demonstrated in bile duct epithelial cells during chronic infection. The infection of resident hepatic stem cells by HBV and HCV could explain the subsequent transmission of the viruses to both hepatocytes and cholangiocytes. However, the hypotheses of a direct mutagen role of HBV and HCV on liver stem cells it is not actually sustained by the existent literature. The role of hepatitis viruses as risk factors for IH-CCA, independently of liver cirrhosis, needs to be better addressed together with the pathogenic mechanism responsible for this association. The magnitude of the association of the HBV and HCV positivity (with or without liver diseases) with IH-CCA is markedly lower than to the association with HCC (61,62) and the same is evident for hemochromatosis (63). However, while HCC arises from cells directly infected by HCV or HBV (resident stem cells or adult hepatocytes), the origin of IH-CCA is likely more heterogeneous, emerging from cell directly infected or cell indirectly affected by Hepatic damage, including resident Hepatic stem cells, cholangiocytes located in different sized bile ducts and cells located in PBGs (64,65). Therefore, when the association of HBV or HCV with IH-CCA is calculated computing the total cases of IH-CCA irrespective of their histogenesis, this certainly attenuate the magnitude of the association. It should be noted that the mortality rates of all types of liver cancer (including liver cancers of poorly specified morphology) increased in the decades 1980-2000 and the improved diagnostic tools are not the only possible explanation for this epidemiologic observation (50) while, in contrast, the burden of hepatitis viruses is a more convincing reason. It should further be noted that viral hepatitis prevention or management approach probably affects the picture of the CCA worldwide incidence. Indeed, efforts in diagnosis and therapies of HCV and HBV related liver diseases had strongly impact on cirrhosis development and HCC emergence (66) but, unfortunately, only industrialized countries are currently able to underwent public health programs aimed to contrast HCV related morbidity for the high cost of prevention, diagnosis, follow-up and treatment. On the light of these considerations, if we consider IH-CCA as a sequelal of chronic viral hepatitis, we could explain why areas of high HCV prevalence such AS Japan (>2.9 % HCV+, IH-CCA incidence=1.25-1.3) are registering less CCA cases with respect to developing countries with same rate of HCV prevalence such as example Korea (>2.9 % HCV+, IH-CC incidence=3.9-4.5). Analyzing the liver diseases related deaths occurring from 1990 through 1998 in the US population (67), the age-specific and age-adjusted mortality rates for hepatitis C-related diseases increased by 220% from 1993 to 1998 (0.57 to 1.67/100 000). As described by Welzel et al. (6), in the US, trough 1992 to 2000, the incidence of IH-CCA increased 4% annually. Thus, the increase in IH-CCAs incidence overlaps geographically and temporally with the increase of the mortality rates for HCV-related liver diseases. At the same time, it is unlikely that HBV infection could play a role in the increased mortality rates for IH-CC, as HBV infection rates and mortality remained stable or slightly decreased in the US population in the last decades (66,68). As far as the other risk factor is concerned, liver flukes are virtually absent in western countries and PSC incidence seems to be stable. Therefore, the reported progressive worldwide increase in incidence and mortality for IH-CCA could be reasonably ascribed to the burden of HCV infection. More than 170 million people are chronically infected with HCV worldwide, and there are 3–4 million new cases of infection each year. Therefore, preventing viral hepatitis infection is a key strategy for IH-CCA prevention. Clear evidence exists that antiviral treatment of acute HCV infection reduces progression of chronic hepatitis C (68,70), and by inference, this would be expected to lower the risk of liver cancers. Pooling of literature data, a protective effect of interferon on HCC development in patients with HCV-related cirrhosis clearly emerges (71,72). Due to anti-necroinflammatory effect and suppression of viral replication, treatment with interferon is estimated to reduce approximately by 50% the annual incidence of HCC in HCV-related chronic hepatitis C and cirrhotic (71). The same positive effect could be exerted on IH-CCA incidence, but this hypothesis should need to be prospectively evaluated. Since 1982 there is a safe and effective HBV vaccine, but due to the cost (2USD/dose), more than 20 years are need to extend worldwide vaccination (73). At 2006, 164 countries have vaccinated their infants against HBV in national immunization programs, compared to 31 countries in 1992 (74). In Taiwan the universal vaccination program against HBV started in the 1980s resulted, a decade later, in reduction of HCC incidence rates in children (75). Similarly a gradual and time-related reduction of IH-CCA should be expected in HBV endemic areas after the introduction of HBV vaccination. Also in this case long prospective observations are necessary. PSC is one of the first recognized risk factor for both IH- and EH-CCA but, unfortunately no strategy has been developed for screening, surveillance or prevention of CCA in this population. Whether concomitant inflammatory bowel diseases (IBD) induce additional risk in PSC patient is still matter of debate. In a European multicentre study, the coexistence and duration of IBD significantly increased the risk of CCA in PSC patients (76). In contrast, in a USA study, neither IBD nor its duration confer additional risk of CCA in PSC patients (30). As far as IBD alone is concerned, there are three case-control studies and one cohort study were the association between IBD and CCA has been evaluated, with OR ranging 1.7 to 4.67 but, in these studies, IBD patients were not controlled for coexistence of PSC (30). Therefore, it is unclear if IBD alone is an independent risk factor for CCA nor if it confers additional risk in PSC patients.
| Definitively estabilished risk factors | Intrahepatic | Extrahepatic |
| Liver flukes (Clonorchis Sinensis, Opisthorchis viverrinii) | ||
| Primary Sclerosing Colangitis | ||
| Choledochal cysts | ||
| Toxins (Thorotrast, dioxins) | ||
| Pancreaticobiliary maljunction with bile duct dilatation | X | |
| Hepatolithiasis | X | |
| Hepatitis C virus infection | X | |
| Probable risk factors | Intrahepatic | Extrahepatic |
| Diabetes, Obesity, Alcohol, Tobacco Smoking | ||
| Genetic Polymorphisms | ||
| Caroli’s Disease | ||
| Inflammatory bowel disease | ||
| Cholangitis and choledocolitiasis | ||
| Surgical biliary-enteric drainage | ||
| Cholecystectomy | X | |
| Cholelithiasis | X | |
| Hepatic Schistosomiasis | X | |
| Liver cirrhosis | X | |
| Hepatitis B virus infection | X | |
| X=risk factors exclusive for intrahepatic-CCA or extrahepatic-CCA ; others factors are common for both CCA types | ||
Conclusions and future perspectives
CCA is a heterogeneous devastating cancer with a worldwide increasing incidence and mortality. The main risk factors are liver flukes in Asian countries, PSC and HCV in western countries. The IH-CCA frequently emerges in the setting of chronic liver diseases requiring differential diagnosis with respect to HCC. It is difficult to diagnose and frequently presents at a late stage when no effective therapeutic intervention is possible. Although much work still remain to be done, significant advance has been obtained in definition of CCA risk factors. However, for a real progress, this should be translated in preventive strategies but, unfortunately, very few or nothing has been so far performed in this setting. In areas endemic for liver flukes, control of foodborne infection or mass anthelmintic therapy certainly will results in decrease of CCA incidence but this require politic and socio-economic interventions. In patients with PBH with bile duct dilatation, the prophylactic excision of gallbladder and common bile duct has been indicated by the Japan Societies Guidelines. In PSC patients a strict follow-up is the unique possible strategy although, in a recent study, performing ultra sounds+CA19-9 annually, resulted in a 91% sensitivity and 62% specificity of CCA diagnosis with at least 2/3 of CCA diagnosed in a stage susceptible of surgical resection (77). Identification of biomarkers in serum and bile for early diagnosis and surveillance of populations at risk and the complete definition of risk factors are the challenges for the next future that could improve the prognosis of this cancer (78).
Acknowledgements
E. Gaudio was supported by research project grant from the University “Sapienza” of Rome and FIRB grant # RBAP10Z7FS_001 and by PRIN grant # 2009X84L84_001. D. Alvaro was supported by FIRB grant # RBAP10Z7FS_004 and by PRIN grant # 2009X84L84_002. The study was also supported by Consorzio Interuniversitario Trapianti d'Organo, Rome, Italy.
References
- Patel T; Medscape. Cholangiocarcinoma--controversies and challenges. Nat Rev Gastroenterol Hepatol 2011;8:189-200.[LinkOut]
- Blechacz B, Komuta M, Roskams T, et al. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;8:512-22.[LinkOut]
- Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford) 2008;10:77-82.[LinkOut]
- Shin HR, Oh JK, Masuyer E, et al. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci 2010;101:579-85.[LinkOut]
- Alvaro D, Crocetti E, Ferretti S, et al. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis 2010;42:490-5.[LinkOut]
- Welzel TM, McGlynn KA, Hsing AW, et al. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst 2006;98:873-5.[LinkOut]
- RARECARE. Training Course on Rare Solid Cancers.[LinkOut]
- West J, Wood H, Logan RF, et al. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer 2006;94:1751-8.[LinkOut]
- Fukukura Y, Taguchi J, Nakashima O, et al. Combined hepatocellular and cholangiocarcinoma: correlation between CT findings and clinicopathological features. J Comput Assist Tomogr 1997;21:52-8.[LinkOut]
- Zuo HQ, Yan LN, Zeng Y, et al. Clinicopathological characteristics of 15 patients with combined hepatocellular carcinoma and cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 2007;6:161-5.[LinkOut]
- Poomphakwaen K, Promthet S, Kamsa-Ard S, et al. Risk factors for cholangiocarcinoma in Khon Kaen, Thailand: a nested case-control study. Asian Pac J Cancer Prev 2009;10:251-8.[LinkOut]
- Shin HR, Oh JK, Masuyer E, et al. Comparison of incidence of intrahepatic and extrahepatic cholangiocarcinoma--focus on East and South-Eastern Asia. Asian Pac J Cancer Prev 2010;11:1159-66.[LinkOut]
- Sripa B, Kaewkes S, Sithithaworn P, et al. Liver fluke induces cholangiocarcinoma. PLoS Med 2007;4:e201.[LinkOut]
- Jin F, Devesa SS, Zheng W, et al. Cancer incidence trends in urban Shanghai, 1972-1989. Int J Cancer 1993;53:764-70.[LinkOut]
- Shin HR, Oh JK, Lim MK, et al. Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in Korea. J Korean Med Sci 2010;25:1011-6.[LinkOut]
- Globocan 2008
- Luke C, Price T, Roder D. Epidemiology of cancer of the liver and intrahepatic bile ducts in an Australian population. Asian Pac J Cancer Prev 2010;11:1479-85.[LinkOut]
- Parkin DM, Ohshima H, Srivatanakul P, et al. Cholangiocarcinoma: epidemiology, mechanisms of carcinogenesis and prevention. Cancer Epidemiol Biomarkers Prev 1993;2:537-44.[LinkOut]
- Jepsen P, Vilstrup H, Tarone RE, et al. Incidence rates of intra- and extrahepatic cholangiocarcinomas in Denmark from 1978 through 2002. J Natl Cancer Inst 2007;99:895-7.[LinkOut]
- Montomoli J, Erichsen R, Nørgaard M, et al. Survival of patients with primary liver cancer in central and northern Denmark, 1998-2009. Clin Epidemiol 2011;3:3-10.[LinkOut]
- Lepage C, Cottet V, Chauvenet M, et al. Trends in the incidence and management of biliary tract cancer: a French population-based study. J Hepatol 2011;54:306-10.[LinkOut]
- West J, Wood H, Logan RF, et al. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer 2006;94:1751-8.[LinkOut]
- Nakanuma Y, Sato Y, Harada K, et al. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol 2010;2:419-27.[LinkOut]
- Komuta M, Spee B, Vander Borght S, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology 2008;47:1544-56.[LinkOut]
- He XR, Wu XP. Difference in biological characteristics and sensitivity to chemotherapy and radiotherapy between intrahepatic and extrahepatic cholangiocarcinoma cells in vitro. Chin Med Sci J 2008;23:54-9.[LinkOut]
- Guedj N, Zhan Q, Perigny M, et al. Comparative protein expression profiles of hilar and peripheral hepatic cholangiocarcinomas. J Hepatol 2009;51:93-101.[LinkOut]
- Komuta M, Govaere O, Vander Borght S, et al. Histological diversity in cholangiocellular carcinoma suggesting different cells of origin: intrahepatic progenitor cells versus hilar mucin producing cells [Abstract]. Journal of Hepatology 2011;54:S37.[LinkOut]
- Aishima S, Kuroda Y, Nishihara Y, et al. Proposal of progression model for intrahepatic cholangiocarcinoma: clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol 2007;31:1059-67.[LinkOut]
- Cardinale V, Semeraro R, Torrice A, et al. Intra-hepatic and extra-hepatic cholangiocarcinoma: New insight into epidemiology and risk factors. World J Gastrointest Oncol 2010;2:407-16.[LinkOut]
- Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54:173-84.[LinkOut]
- Terada T, Nakanuma Y. Pathological observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers. III. Survey of necroinflammation and cystic dilatation. Hepatology 1990;12:1229-33.[LinkOut]
- Terada T, Nakanuma Y. Pathologic observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers: IV. Hyperplasia of intramural and extramural glands. Hum Pathol 1992;23:483-90.[LinkOut]
- Terada T, Ashida K, Endo K, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology 1998;33:325-31.[LinkOut]
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol 1998;29:175-80.[LinkOut]
- Cardinale V, Wang Y, Carpino G, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes and pancreatic islets. Hepatology. 2011 Aug 1. [Epub ahead of print][LinkOut]
- Matsubara T, Sakurai Y, Zhi LZ, et al. K-ras and p53 gene mutations in noncancerous biliary lesions of patients with pancreaticobiliary maljunction. J Hepatobiliary Pancreat Surg 2002;9:312-21.[LinkOut]
- Miyazaki M, Takada T, Miyakawa S, et al. Risk factors for biliary tract and ampullary carcinomas and prophylactic surgery for these factors. J Hepatobiliary Pancreat Surg 2008;15:15-24.[LinkOut]
- Chijiiwa K, Kimura H, Tanaka M. Malignant potential of the gallbladder in patients with anomalous pancreaticobiliary ductal junction. The difference in risk between patients with and without choledochal cyst. Int Surg 1995;80:61-4.[LinkOut]
- Yamao K, Mizutani S, Nakazawa S, et al. Prospective study of the detection of anomalous connections of pancreatobiliary ducts during routine medical examinations. Hepatogastroenterology 1996;43:1238-45.[LinkOut]
- Lipsett PA, Pitt HA, Colombani PM, et al. Choledochal cyst disease. A changing pattern of presentation. Ann Surg 1994;220:644-52.[LinkOut]
- Gaudio E, Carpino G, Cardinale V, et al. New insights into liver stem cells. Dig Liver Dis 2009;41:455-62.[LinkOut]
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105-11.[LinkOut]
- Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene 2006;25:3818-22.[LinkOut]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008;8:755-68.[LinkOut]
- Visvader JE. Cells of origin in cancer. Nature 2011;469:314-22.[LinkOut]
- Carpino G, Cardinale V, Onori P, et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat 2011; in press.[LinkOut]
- Kimura W, Futakawa N, Zhao B. Neoplastic diseases of the papilla of Vater. J Hepatobiliary Pancreat Surg 2004;11:223-31.[LinkOut]
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006;118:3030-44.[LinkOut]
- Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis 2005 Sep;5:558-67.[LinkOut]
- McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev 2006;15:1198-203.[LinkOut]
- El-Serag HB, Engels EA, Landgren O, et al. Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: A population-based study of U.S. veterans. Hepatology 2009;49:116-23.[LinkOut]
- Yamamoto S, Kubo S, Hai S, et al.Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci 2004;95:592-5.[LinkOut]
- Tanaka M, Tanaka H, Tsukuma H, et al. Risk factors for intrahepatic cholangiocarcinoma: a possible role of hepatitis B virus. J Viral Hepat 2010;17:742-8.[LinkOut]
- Fwu CW, Chien YC, You SL, et al. Hepatitis B virus infection and risk of intrahepatic cholangiocarcinoma and non-Hodgkin lymphoma: a cohort study of parous women in Taiwan. Hepatology 2011;53:1217-25.[LinkOut]
- Lee CH, Chang CJ, Lin YJ, et al. Viral hepatitis-associated intrahepatic cholangiocarcinoma shares common disease processes with hepatocellular carcinoma. Br J Cancer 2009;100:1765-70.[LinkOut]
- Tao LY, He XD, Qu Q, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a case-control study in China. Liver Int 2010;30:215-21.[LinkOut]
- Zhou H, Wang H, Zhou D, et al. Hepatitis B virus-associated intrahepatic cholangiocarcinoma and hepatocellular carcinoma may hold common disease process for carcinogenesis. Eur J Cancer 2010;46:1056-61.[LinkOut]
- Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev 2005;1:253-60.[LinkOut]
- Nicoll AJ, Angus PW, Chou ST, et al. Demonstration of duck hepatitis B virus in bile duct epithelial cells: implications for pathogenesis and persistent infection. Hepatology 1997;25:463-9.[LinkOut]
- Haruna Y, Kanda T, Honda M, et al. Detection of hepatitis C virus in the bile and bile duct epithelial cells of hepatitis C virus-infected patients. Hepatology 2001;33:977-80.[LinkOut]
- Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer 1998;75:347-54.[LinkOut]
- Shi J, Zhu L, Liu S, et al. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer 2005;92:607-12.[LinkOut]
- Ellervik C, Birgens H, Tybjaerg-Hansen A, et al. Hemochromatosis genotypes and risk of 31 disease endpoints: meta-analyses including 66,000 cases and 226,000 controls. Hepatology 2007;46:1071-80.[LinkOut]
- Callea F, Sergi C, Fabbretti G, et al. Precancerous lesions of the biliary tree. J Surg Oncol Suppl 1993;3:131-3.[LinkOut]
- Nakanuma Y, Minato H, Kida T, et al. Pathology of cholangiocellular carcinoma. 1994;p39–50. In: Tobe T, Kameda H, Okudaira M, Ohto M, editors. Primary liver cancer in Japan. Springer-Verlag, Tokyo, Japan.
- De Flora S, Bonanni P. The prevention of infection-associated cancers. Carcinogenesis 2011;32:787-95.[LinkOut]
- Vong S, Bell BP. Chronic liver disease mortality in the United States, 1990-1998. Hepatology 2004;39:476-83.[LinkOut]
- McQuillan GM, Coleman PJ, Kruszon-Moran D, et al. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health 1999;89:14-8.[LinkOut]
- Kamal SM. Acute hepatitis C: a systematic review. Am J Gastroenterol 2008;103:1283-97.[LinkOut]
- Dore GJ, Hellard M, Matthews GV, et al. Effective treatment of injecting drug users with recently acquired hepatitis C virus infection. Gastroenterology 2010;138:123-35.[LinkOut]
- Castello G, Costantini S, Scala S. Targeting the inflammation in HCV-associated hepatocellular carcinoma: a role in the prevention and treatment. J Transl Med 2010;8:109.[LinkOut]
- Miyake Y, Takaki A, Iwasaki Y, et al. Meta-analysis: interferon-alpha prevents the recurrence after curative treatment of hepatitis C virus-related hepatocellular carcinoma. J Viral Hepat 2010;17:287-92.[LinkOut]
- Hepatitis B vaccines. Wkly Epidemiol Rec 2004;79:255-63.[LinkOut]
- WHO. Hepatitis B. 2008 (Fact sheet No. 204) [Accessed Nov 10th 2009]. Aviable online: http://www.who.int/mediacentre/factsheets/fs204/en/.
- Chang MH, Chen CJ, Lai MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med 1997;336:1855-9.[LinkOut]
- Erichsen R, Jepsen P, Vilstrup H, et al. Incidence and prognosis of cholangiocarcinoma in Danish patients with and without inflammatory bowel disease: a national cohort study, 1978-2003. Eur J Epidemiol 2009;24:513-20.[LinkOut]
- Charatcharoenwitthaya P, Enders FB, Halling KC, et al. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology 2008;48:1106-17.[LinkOut]
- Alvaro D. Progranulin and cholangiocarcinoma: another bad boy on the block! Gut 2011. [Epub ahead of print][LinkOut]


