Mechanisms for nicotine in the development and progression of gastrointestinal cancers
Abstract
Long-term smoking is major risk factor for a variety of cancers, including those of the gastrointestinal (GI) tract. Historically, nicotine and its derivatives are well known for their role in addiction, and have more recently been documented for their carcinogenic role in a number of human cancers. The cellular and molecular pathways activated by nicotine mimic physiological and environmental carcinogenesis in cancers throughout the GI tract potentiating cancer growth and/or inducing the formation of cancer on their own. Thus, it is important to unlock the carcinogenic mechanisms induced by nicotine in these systems, and underscore nicotine’s potential as an environmental hazard. This review outlines the specific pathways demonstrated to mediate nicotine’s carcinogenic mechanism in the GI tract. The abundance of cell and animal evidence calls for increased epidemiologic and case-control evaluation of nicotine’s role in cancer.
Key words
INicotine; nitrosamines; gastrointestinal neoplasms; smoking; catecholamines; nicotinic acetylcholine receptors; carcinogen; autocrine signaling; angiogenesis; proliferation; migration
Introduction
The World Health Organization (WHO) reports that in the 20th century, tobacco abuse resulted in the deaths of one hundred million people, and the deaths of one billion this next century if abuse is left unabated (1). Currently, there are more tobacco-related deaths than for AIDS, tuberculosis and malaria combined. In the United States, over 400,000 cancer-deaths are caused each year by cigarette smoke (2). It is well-known that cigarette smoke increases the risk of chronic obstructive pulmonary disease, heart disease, and lung and esophageal cancers (3-6). Cigarette smoke is also known for increasing the risk of cancers throughout the gastrointestinal (GI) tract. It is the strongest risk factor for gastric cancers in Taiwan and increases tumorigenesis in the intestinal tract, liver, and colon (4,7-9). In the pancreas, there is strong evidence from epidemiological and case control studies showing that cigarette smoke is a major, if not the most important risk factor for pancreatic cancer (10-23).
Cigarette smoke contains multiple compounds that exert effects on the GI tract. Of these compounds, nicotine has been extensively studied for its biological effects on mood, appetite, and task performance; and owing to its interaction with nicotinic receptors in the brain, is highly addictive (24-26). Nicotine is rapidly absorbed through the skin, alveoli, and the GI tract and distributed rapidly throughout the body. The nicotine metabolites 4-methylnitrosamino-1-3-pyridyl-1-butanone (NNK), and N’nitrosonornicotine (NNN), are particularly known for their carcinogenic properties (27-29). In rats, NNN has been identified as a causative agent of esophageal cancer (30). In adenocarcinoma cells of the colon, NNK promotes the upregulation of nuclear factor-kappa B(NF-κB) binding and an increase in the cancer-promoting homomeric nicotinic acetylcholine receptor (nAChR), α7-nAChR (31).
nAChRs are ligand-gated transmembrane ion channel receptors that bind and respond to nicotine (32). Twelve genes encode the nAChRs, which are discretely expressed throughout various functional areas of the CNS (27,33,34). Upon nicotine binding, the conformation of the nAChR subunits changes to open a central pore and allow the flow of ions such as calcium (Ca2+). Ca2+ entry reduces the negative charge across the plasma membrane (depolarization) resulting in activation of the voltage-gated Ca2+ channels and further Ca2+ influx. This process results in activation of downstream pathways and neurotransmitter release. Until recently, it was thought that nAChRs exist only in the nervous system. However, increasing evidence suggests that nAChRs are expressed in virtually all mammalian cells and are evolutionarily conserved (35). Outside of the nervous system, nAChRs regulate a complex network of stimulatory and inhibitory signals that regulate a number of processes such as growth, angiogenesis, neurogenic release of factors, and modulation of the microenvironment (36). While different members of the nAChR family may trigger converging signaling pathways, they often have diverse and even opposing actions. The two most important and evolutionarily-conserved of these are the α7-homomeric nicotinic receptor (α7-nAChR), and the α4/β2-heteromeric nicotinic receptor (α4β2-nAChR), which cooperatively promote breast and prostate cancer by increasing dopamine synthesis (36). On the other hand, in most cancers, these receptors have opposing actions. In the lung, α7- nAChR promotes cancer by increasing catecholamine synthesis, and α4/β2-nAChR suppresses catecholamine-induced cancer progression by GABA release (36).
There is increasing evidence that nicotine and its derivatives activate and promote key processes in the carcinogenesis of GI cancers (9). Over the past two decades, experimental models using animals, cell cultures, and clinical data have identified nicotine as a carcinogen for virtually all GI organs (9). Although other chemicals found in cigarette smoke contribute to the development of cancer, we focus on nicotine in this review.
Role of nicotine in esophageal and oral cancer
In vitro experiments on squamous epithelium give insight into the carcinogenic pathways nicotine induces to promote esophageal and oral cancers. Esophageal and oral cancer is commonly promoted by an epithelial-mesenchymal transition (EMT) of the keratinocyte lining (squamous epithelium) of the esophagus. In esophageal keratinocyte culture, nicotine has been shown to cause structural alterations in nAChRs by displacing the local neurotransmitter Ach (37). In this study, nicotine caused a downstream change in the transcriptional/translational control of cell cycle and differentiation genes including Ki-67, PCNA, p21, cyclin D1, and p53 (37). A change in the expression of nAChR subunits α3, α5, α7, and β2, and β4 was also induced by nicotine as demonstrated by RT-PCR and immunoblotting (37). Multiple studies have demonstrated the oncogenic roles of the α7-nAChRs in keratinocyte cultures (38-42). Chernyavsky et al. studied the carcinogenic mechanisms of nAChR-receptor activation and demonstrated increased NF-κB and SLURP1 activity and eferession, and changes in keratinocyte morphology, following α7-nAChR stimulation (40). Nicotine displays oncogenic activity by triggering increased production and release of growth factors. Zong et al. demonstrated that nicotine increases COX-2 and MMP2 activity, and enhances cell migration and invasion (42). Additionally, nicotine alters fragile histidine triad gene (FHIT) gene expression through methylation. In cellular models of squamous cell carcinoma (SCC), promoter methylation of tumor suppressor p16 and FHIT induces the development of squamous cell carcinoma of the esophagus (43-45). Methylation of the FHIT promoter in nicotine-treated cells conferred a growth and proliferative advantage through altered expression of genes such as anti-apoptotic genes p53 and p16 (43-45). Furthermore, re-expression of FHIT protein was seen in esophageal squamous epithelial cells following smoking cessation of nicotine exposure (43-45).
Role of nicotine in gastric cancer
Nicotine is a major risk factor for gastric cancer (46). In the recent years, in vitro investigation into inflammatory messengers and growth signals has provided mechanistic information about nicotine’s carcinogenic properties in the stomach. The pioneering studies performed by Dr. Cho’s laboratory have provided data on the signaling pathways of nicotine uses to promote gastric cancer growth. Shin and Cho demonstrated that nicotine increases the proliferation and migration of gastric cancer cells by inducing COX-2, prostaglandin E2, VEGF release, and activates ERK, and stimulates cancer cell angiogenesis through a COX-2-dependent increase in VEGF and VEGF receptor (2,47-50). These studies are intriguing since the same signaling pathways mediate the formation of gastric cancer by the bacterium Helicobacter pylori (33,34). The ERK-mediated 5-Lipoxygenase (5-LOX) pathway was also investigated for its role in the pathogenesis of gastric cancer cells (49). Here, nicotine induced 5-LOX expression to promote cell growth and invasion (49). Inhibiting 5-LOX using the protein inhibitor MK886 caused a decrease in invasion and triggered cell cycle arrest by decreasing the relative transcription of cyclin D1, Cyclin E, p-RB, DP-1, and E2F (49). Additionally, 5-LOX blockade resulted in the inactivation of E-cadherin and activation of transcriptional repressor Snail, indicating an epithelial-mesenchymal transition (EMT) (49). In support of this, a recent study demonstrated an increased number of migratory cells during nicotine stimulation in a transwell migration assay (51). Nicotine was shown to induce a marked downregulation of E cadherin and upregulation of ZEB-1 and Snail (51). Nicotine has also been shown to decrease the expression of anti-proliferative genes through induction of a microRNA (miRNA) pathway. One group determined that nicotine regulates the expression of microRNAs (miRNA) 16 and 21 in gastric cancer cells, through activation of NF-κB by prostaglandin E receptors (EP2 and EP4) (52). Knockdown of NF-κB markedly reduced cell proliferation, and miRNA-16/21 expression; and knockdown of EP2 and EP4 impaired the NF- κB/miRNA pathway (52).
Early in vivo work showed that long-term administration of nicotine to rats increased gastric cancer incidence and caused the initiation of gastric tumors at an early stage (53). A more recent follow-up study by Shin et al. performed using human gastric xenografts on rats, showed increased expression of ERK, COX-2, and VEGF following nicotine administration (54). Reducing the expression of COX-2 attenuated tumor growth and neovascularization (54).
Clinical evidence for human markers of gastric cancer has been presented in association studies on gastric cancer patients. A study investigating the association between different TNF genetic variants and cancer induced by smoking, found a significant association between TNF-alpha-857 genetic variant and gastric cancer which was propounded by smoking (55). Another study found a correlation between clinical evidence of tumor severity (e.g. invasion of tumor, metastasis, and clinical stage) and uPAR expression in gastric cancer patients (56). Since nicotine increases the expression of uPA/uPAR, it is likely that uPAR could be another clinical key carcinogenic player induced by nicotine (49).
Role of nicotine in colon cancer
Key processes for the progression of colon cancer including proliferation, migration, angiogenesis, and escape from apoptosis have been demonstrated by in vitro treatment of nicotine. Nicotine has been shown to increase proliferation by changing the expression of receptors and their phosphorylation patterns in several different mitogenic pathways. One study showed enhanced phosphorylation of epidermal growth factor receptor (EGFR) and increased expression of 5-LOX in colon cancer cells following nicotine exposure (31). By blocking these receptors (EGFR and/or 5-LOX), the proliferation induced by nicotine stimulation was inhibited. Similarly, the upregulation of acetylcholine and noradrenaline receptors was induced by nicotine and enhanced the proliferation of colorectal carcinoma cells (57,58). Nicotine was shown to increase the expression of β1- and β2-adrenoceptors causing increased expression/production of VEGF, COX-2, PGE2 and micro vessel densities. Inhibition of β-adrenergic receptors by atenolol abrogated these effects (57,58). Additional studies have demonstrated that nicotine/smoking stimulates angiogenesis and neovascularization in colon cancer through increases in VEGF, 5-LOX, COX-2, and MMP2/9 (57-60). A nicotine/ α7-nAChR pathway has been shown to play an important role in colon cancer cell migration by induction of fibronectin (61). In this report, nicotine and COX-2 were necessary for induction of fibronectin as demonstrated by siRNA knockdown and chemical inhibition of COX-2 and α7- nAChR (61). Since nicotine increases growth factor synthesis and receptor expression, autocrine signaling may likely be another important oncogenic mechanism for the development and progression of colon cancer. Although this likely occurs through some of the pathways previously discussed, an autocrine pathway for nicotine carcinogenesis has been proposed in human colon cancer cells for SLURP-1 (62). This short study reported that nicotine induces a decrease in the release of an antiproliferative autocrine peptide SLURP-1, an endogenous α7-nAChR ligand.
Role of nicotine in pancreatic cancer
Studies have demonstrated that nicotine works through various mechanisms to activate key mitogenic MAPK cascades in the development of pancreatic cancer (63). The activation of receptors and/or cellular targets for nicotine results in downstream phosphorylation of MAPKs including p42, JNK, and ERK 1/2 resulting in the induction of mitogenic transcription factors such as ELK 1 (63-65). One working model depicting the development of pancreatic adenocarcinoma by chronic nicotine exposure elegantly diagramed an important pathway leading to angiogenesis, proliferation and migration, and apoptosis (66). In this model, nicotine causes a change in the expression of nicotinic receptors, increasing α7-nAChR and decreasing α4β2-nAChr; leading to increased noradrenaline, and decreased GABA, respectively (66). This contributes to cellular changes by increasing β-adrenergic activation of CREB, ERK 1/2, and AKT, and decreasing GABA an inhibitor of adenylyl cyclase activity a key regulator of MAPK activity (66). From a signaling perspective, this pathway is analogous to the addiction pathway induced by nicotine in the brain and in a number of cancers including small-cell lung cancer SCLC and pulmonary neural endocrine cells (36). A pathway diverging from the α7 nAChR has also been described (67). This study demonstrated that NNK stimulation of α7-nAChR transactivated the EGFR receptor, increasing cAMP and ERK 1/2 in immortalized pancreatic duct cells (67).
In addition to changing the expression levels of surface receptors and intracellular signal peptides, nicotine induces the progression of pancreatic cancer by stimulating increases in autocrine/paracrine secretion. One group investigating the effect of nicotine on pancreatic adenocarcinoma cell migration and angiogenesis, demonstrated that nicotine induced osteopontin (OPN) mRNA expression mRNA (68). Follow-up work by the same group showed nicotine increased MMP-9 and VEGF mRNA expression, which was dependent on osteopontin (69). In a similar fashion, the pro-inflammatory cytokine, monocyte chemoattractant protein (MCP-1), was induced and secreted upon nicotine exposure, as shown by RT-PCR, and ELISA, and appeared to colocalize with OPN in confocal images (70). Furthermore, IHC staining in human pancreatic tissue showed colocalization of MCP-1 and OPN in malignant ducts compared to normal pancreatic ducts (70).
Role of nicotine in liver carcinogenesis
In comparison to other cancers of the GI tract, there is limited information about nicotine’s effects on liver carcinogenesis. While smoking is not listed as a major risk factor for hepatocellular carcinoma (HCC), it is to a lesser degree associated with HCC by itself, and profoundly associated with HCC in certain liver conditions and/or diseases (71,72). For example, studies on patients with primary sclerosing cholangitis (PSC), have reported a significant association between HCC and smoking/nicotine abuse (73,74). In vivo models have demonstrated that nicotine derivatives act as liver carcinogens. One study evaluating the carcinogenicity of nitrosamine derivatives found that subcutaneous injection of NNK induced lung and liver tumors in rats (75).
Summary
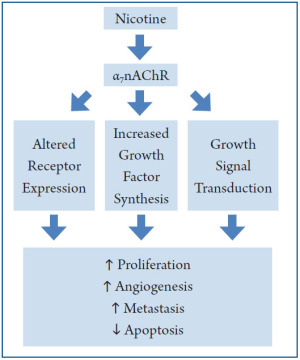
A number of factors controlled by cholinergic and adrenergic signaling plays critical roles in determining the pathophysiology of the GI tract. While it is important to remember that many endogenous and environmental signals trigger carcinogenic mechanisms in the GI, we emphasized the mechanisms (Figure 1) nicotine and its derivatives trigger in experimental models of cancer. First, nicotine-signaling potently modulates the cell’s receptor profile at the plasma membrane. Chronic nicotine exposure causes an increase in α7-nAChR and a decrease in α4β2-nAChR expression. While the α7-nAChR promotes cancer through increased catecholamine production, the α4β2-nAChR suppresses the development of cancer via GABA inhibition of β-adrenergic signaling (36). A similar increase in expression of β-adrenergic receptors and the non-neuronal receptors including uPAR, VEGFR, Prostaglandin receptors and EGFR is triggered by nicotine exposure. Second, nicotine induces synthesis of hormones and cytokines important for the growth, metastasis, and invasion of cancer. This is exemplified by VEGF, which mediates the growth of cancer arising in nearly all GI organs; and the pro-inflammatory cytokine COX-2, most known for its role in the development of gastric cancer. Finally, nicotine and its derivatives may themselves activate mitogenic pathways such as the MAPKs, either directly, or through signal-transduction by cholinergic signaling.
Perspective and future directions
While nicotine supports the growth of GI cancers in cells and animal models, there is insufficient epidemiological evidence that nicotine by itself causes cancer in humans. One study reported five-year surveillance data of 3,320 Canadian patients taking nicotine-replacement therapy (NRT) and found that usage of the drug could not predict the occurrence of cancer in this population (76). Thus, there is a need for further studies with greater than five years follow-up. Since GI disturbances are the most common adverse effect in NRT trials, it will be interesting to learn whether long-term NRT is associated with increased GI cancer risk (77). Moreover, since nicotine acts through some of the same processes, which promote other types of cancer, it is likely nicotine will accelerate the growth of cancer in co-morbid conditions of the GI tract.
Acknowledgments
Portions of this work was supported by a NIH RO1 Grant (DK081442) and a Department of Veteran Affairs, Career Development-2 grant awarded to Shannon Glaser.
References
- Organization WH. WHO Report on the Global Tobacco Epidemic, 2011. The mPower Package;2011.
- Shin VY, Cho CH. Nicotine and gastric cancer. Alcohol 2005;35:259-64.[LinkOut]
- Devereux G. ABC of chronic obstructive pulmonary disease. Definition, epidemiology, and risk factors. BMJ 2006;332:1142-4.[LinkOut]
- Liu ES, Ye YN, Shin VY, et al. Cigarette smoke exposure increases ulcerative colitis-associated colonic adenoma formation in mice. Carcinogenesis 2003;24:1407-13.[LinkOut]
- Tsiara S, Elisaf M, Mikhailidis DP. Influence of smoking on predictors of vascular disease. Angiology 2003;54:507-30.[LinkOut]
- Shopland DR. Tobacco use and its contribution to early cancer mortality with a special emphasis on cigarette smoking. Environ Health Perspect 1995;103:131-42.[LinkOut]
- Chen MJ, Chiou YY, Wu DC, et al. Lifestyle habits and gastric cancer in a hospital-based case-control study in Taiwan. Am J Gastroenterol 2000;95:3242-9.[LinkOut]
- Huggett MT, Pereira SP. Diagnosing and managing pancreatic cancer. Practitioner 2011;255:21-5,2-3.[LinkOut]
- Thun MJ, Henley SJ, Calle EE. Tobacco use and cancer: an epidemiologic perspective for geneticists. Oncogene 2002;21:7307-25.[LinkOut]
- Novello AC. Surgeon General’s report on the health benefits of smoking cessation. Public Health Rep 1990;105:545-8.[LinkOut]
- Howe GR, Jain M, Burch JD, et al. Cigarette smoking and cancer of the pancreas: evidence from a population-based case-control study in Toronto, Canada. Int J Cancer 1991;47:323-8.[LinkOut]
- Olsen GW, Mandel JS, Gibson RW, et al. A case-control study of pancreatic cancer and cigarettes, alcohol, coffee and diet. Am J Public Health 1989;79:1016-9.[LinkOut]
- Wynder EL, Mabuchi K, Maruchi N, et al. Epidemiology of cancer of the pancreas. J Natl Cancer Inst 1973;50:645-67.[LinkOut]
- Fernandez E, La Vecchia C, Porta M, et al. Pancreatitis and the risk of pancreatic cancer. Pancreas 1995;11:185-9.[LinkOut]
- Mack TM, Yu MC, Hanisch R, et al. Pancreas cancer and smoking, beverage consumption, and past medical history. J Natl Cancer Inst 1986;76:49-60.[LinkOut]
- Silverman DT, Dunn JA, Hoover RN, et al. Cigarette smoking and pancreas cancer: a case-control study based on direct interviews. J Natl Cancer Inst 1994;86:1510-6.[LinkOut]
- Bertuccio P, La Vecchia C, Silverman DT, et al. Cigar and pipe smoking, smokeless tobacco use and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2011;22:1420-6.[LinkOut]
- Bueno de Mesquita HB, Maisonneuve P, Moerman CJ, et al. Life-time history of smoking and exocrine carcinoma of the pancreas: a populationbased case-control study in The Netherlands. Int J Cancer 1991;49:816-22.[LinkOut]
- Zatonski WA, Boyle P, Przewozniak K, et al. Cigarette smoking, alcohol, tea and coffee consumption and pancreas cancer risk: a case-control study from Opole, Poland. Int J Cancer 1993;53:601-7.[LinkOut]
- Rivenson A, Hoffmann D, Prokopczyk B, et al. Induction of lung and exocrine pancreas tumors in F344 rats by tobacco-specific and Arecaderived N-nitrosamines. Cancer Res 1988;48:6912-7.[LinkOut]
- Gold EB, Goldin SB. Epidemiology of and risk factors for pancreatic cancer. Surg Oncol Clin N Am 1998;7:67-91.[LinkOut]
- Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base report on pancreatic cancer. Cancer 1995;76:1671-7.[LinkOut]
- Hirayama T. Epidemiology of pancreatic cancer in Japan. Jpn J Clin Oncol 1989;19:208-15.[LinkOut]
- Cardenas L, Tremblay LK, Naranjo CA, et al. Brain reward system activity in major depression and comorbid nicotine dependence. J Pharmacol Exp Ther 2002;302:1265-71.[LinkOut]
- Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N Engl J Med 1988;319:1318-30.[LinkOut]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2011;CD006103.[LinkOut]
- Wu WK, Cho CH. The pharmacological actions of nicotine on the gastrointestinal tract. J Pharmacol Sci 2004;94:348-58.[LinkOut]
- Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst 1999;91:1194-210.[LinkOut]
- Seitz HK, Cho CH. Contribution of alcohol and tobacco use in gastrointestinal cancer development. Methods Mol Biol 2009;472:217-41.[LinkOut]
- Stoner GD, Adams C, Kresty LA, et al. Inhibition of N’-nitrosonornicotineinduced esophageal tumorigenesis by 3-phenylpropyl isothiocyanate. Carcinogenesis 1998;19:2139-43.[LinkOut]
- Ye YN, Liu ES, Shin VY, et al. Nicotine promoted colon cancer growth via epidermal growth factor receptor, c-Src, and 5-lipoxygenase-mediated signal pathway. J Pharmacol Exp Ther 2004;308:66-72.[LinkOut]
- Dwoskin LP, Smith AM, Wooters TE, et al. Nicotinic receptor-based therapeutics and candidates for smoking cessation. Biochem Pharmacol 2009;78:732-43.[LinkOut]
- Chavez-Noriega LE, Crona JH, Washburn MS, et al. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther 1997;280:346-56.[LinkOut]
- Dineley-Miller K, Patrick J. Gene transcripts for the nicotinic acetylcholine receptor subunit, beta4, are distributed in multiple areas of the rat central nervous system. Brain Res Mol Brain Res 1992;16:339-44.[LinkOut]
- Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol 2008;154:1558-71.[LinkOut]
- Schuller HM. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat Rev Cancer 2009;9:195-205.[LinkOut]
- Arredondo J, Nguyen VT, Chernyavsky AI, et al. A receptor-mediated mechanism of nicotine toxicity in oral keratinocytes. Lab Invest 2001;81:1653-68.[LinkOut]
- Nguyen VT, Hall LL, Gallacher G, et al. Choline acetyltransferase, acetylcholinesterase, and nicotinic acetylcholine receptors of human gingival and esophageal epithelia. J Dent Res 2000;79:939-49.[LinkOut]
- Calleja-Macias IE, Kalantari M, Bernard HU. Cholinergic signaling through nicotinic acetylcholine receptors stimulates the proliferation of cervical cancer cells: an explanation for the molecular role of tobacco smoking in cervical carcinogenesis? Int J Cancer 2009;124:1090-6.[LinkOut]
- Chernyavsky AI, Arredondo J, Galitovskiy V, et al. Upregulation of nuclear factor-kappaB expression by SLURP-1 is mediated by alpha7-nicotinic acetylcholine receptor and involves both ionic events and activation of protein kinases. Am J Physiol Cell Physiol 2010;299:C903-11.[LinkOut]
- Arredondo J, Nguyen VT, Chernyavsky AI, et al. Central role of alpha7 nicotinic receptor in differentiation of the stratified squamous epithelium. J Cell Biol 2002;159:325-36.[LinkOut]
- Zong Y, Zhang ST, Zhu ST. Nicotine enhances migration and invasion of human esophageal squamous carcinoma cells which is inhibited by nimesulide. World J Gastroenterol 2009;15:2500-5.[LinkOut]
- Tanaka H, Shimada Y, Imamura M, et al. Multiple types of aberrations in the p16 (INK4a) and the p15(INK4b) genes in 30 esophageal squamouscell- carcinoma cell lines. Int J Cancer 1997;70:437-42.[LinkOut]
- Tanaka H, Shimada Y, Harada H, et al. Methylation of the 5’ CpG island of the FHIT gene is closely associated with transcriptional inactivation in esophageal squamous cell carcinomas. Cancer Res 1998;58:3429-34.[LinkOut]
- Soma T, Kaganoi J, Kawabe A, et al. Nicotine induces the fragile histidine triad methylation in human esophageal squamous epithelial cells. Int J Cancer 2006;119:1023-7.[LinkOut]
- Hoffmann D. Mechanisms in tobacco carcinogenesis. In: Harris CC, Center B editors. Cold Spring Harbor Laboratory;1986.
- Caputo R, Tuccillo C, Manzo BA, et al. Helicobacter pylori VacA toxin upregulates vascular endothelial growth factor expression in MKN 28 gastric cells through an epidermal growth factor receptor-, cyclooxygenase-2- dependent mechanism. Clin Cancer Res 2003;9:2015-21.[LinkOut]
- Oshima H, Popivanova BK, Oguma K, et al. Activation of epidermal growth factor receptor signaling by the prostaglandin E(2) receptor EP4 pathway during gastric tumorigenesis. Cancer Sci 2011;102:713-9.[LinkOut]
- Shin VY, Jin HC, Ng EK, et al. Activation of 5-lipoxygenase is required for nicotine mediated epithelial-mesenchymal transition and tumor cell growth. Cancer Lett 2010;292:237-45.[LinkOut]
- Shin VY, Wu WK, Chu KM, et al. Nicotine induces cyclooxygenase-2 and vascular endothelial growth factor receptor-2 in association with tumorassociated invasion and angiogenesis in gastric cancer. Mol Cancer Res 2005;3:607-15.[LinkOut]
- Lien YC, Wang W, Kuo LJ, et al. Nicotine promotes cell migration through alpha7 nicotinic acetylcholine receptor in gastric cancer cells. Ann Surg Oncol 2011;18:2671-9.[LinkOut]
- Shin VY, Jin H, Ng EK, et al. NF-κB targets miR-16 and miR-21 in gastric cancer: involvement of prostaglandin E receptors. Carcinogenesis 2011;32:240-5.[LinkOut]
- Gurkalo VK, Volfson NI. Nicotine influence upon the development of experimental stomach tumors. Arch Geschwulstforsch 1982;52:259-65.[LinkOut]
- Shin VY, Wu WK, Ye YN, et al. Nicotine promotes gastric tumor growth and neovascularization by activating extracellular signal-regulated kinase and cyclooxygenase-2. Carcinogenesis 2004;25:2487-95.[LinkOut]
- Yang JJ, Ko KP, Cho LY, et al. The role of TNF genetic variants and the interaction with cigarette smoking for gastric cancer risk: a nested casecontrol study. BMC Cancer 2009;9:238.[LinkOut]
- Kita Y, Fukagawa T, Mimori K, et al. Expression of uPAR mRNA in peripheral blood is a favourite marker for metastasis in gastric cancer cases. Br J Cancer 2009;100:153-9.[LinkOut]
- Wong HP, Yu L, Lam EK, et al. Nicotine promotes cell proliferation via alpha7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells. Toxicol Appl Pharmacol 2007;221:261-7.[LinkOut]
- Wong HP, Yu L, Lam EK, et al. Nicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activation. Toxicol Sci 2007;97:279-87.[LinkOut]
- Natori T, Sata M, Washida M, et al. Nicotine enhances neovascularization and promotes tumor growth. Mol Cells 2003;16:143-6.[LinkOut]
- Ye YN, Wu WK, Shin VY, et al. A mechanistic study of colon cancer growth promoted by cigarette smoke extract. Eur J Pharmacol 2005;519:52-7.[LinkOut]
- Wei PL, Kuo LJ, Huang MT, et al. Nicotine enhances colon cancer cell migration by induction of fibronectin. Ann Surg Oncol 2011;18:1782-90.[LinkOut]
- Pettersson A, Nylund G, Khorram-Manesh A, et al. Nicotine induced modulation of SLURP-1 expression in human colon cancer cells. Auton Neurosci 2009;148:97-100.[LinkOut]
- Chowdhury P, Udupa KB. Nicotine as a mitogenic stimulus for pancreatic acinar cell proliferation. World J Gastroenterol 2006;12:7428-32.[LinkOut]
- Dabrowski A, Grady T, Logsdon CD, et al. Jun kinases are rapidly activated by cholecystokinin in rat pancreas both in vitro and in vivo. J Biol Chem 1996;271:5686-90.[LinkOut]
- Duan RD, Williams JA. Cholecystokinin rapidly activates mitogen-activated protein kinase in rat pancreatic acini. Am J Physiol 1994;267:G401-8.[LinkOut]
- Schuller HM, Al-Wadei HA. Neurotransmitter receptors as central regulators of pancreatic cancer. Future Oncol 2010;6:221-8.[LinkOut]
- Askari MD, Tsao MS, Schuller HM. The tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone stimulates proliferation of immortalized human pancreatic duct epithelia through beta-adrenergic transactivation of EGF receptors. J Cancer Res Clin Oncol 2005;131:639-48.[LinkOut]
- Chipitsyna G, Gong Q, Anandanadesan R, et al. Induction of osteopontin expression by nicotine and cigarette smoke in the pancreas and pancreatic ductal adenocarcinoma cells. Int J Cancer 2009;125:276-85.[LinkOut]
- Lazar M, Sullivan J, Chipitsyna G, et al. Involvement of osteopontin in the matrix-degrading and proangiogenic changes mediated by nicotine in pancreatic cancer cells. J Gastrointest Surg 2010;14:1566-77.[LinkOut]
- Lazar M, Sullivan J, Chipitsyna G, et al. Induction of monocyte chemoattractant protein-1 by nicotine in pancreatic ductal adenocarcinoma cells: role of osteopontin. Surgery 2010;148:298-309.[LinkOut]
- Marrero JA, Fontana RJ, Fu S, et al. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J Hepatol 2005;42:218-24.[LinkOut]
- Sherman M, Llovet JM. Smoking , hepatitis B v irus infection, and development of hepatocellular carcinoma. J Natl Cancer Inst 2011;103:1642-3.[LinkOut]
- Bergquist A, Glaumann H, Persson B, et al. Risk factors and clinical presentation of hepatobiliary carcinoma in patients with primary sclerosing cholangitis: a case-control study. Hepatology 1998;27:311-6.[LinkOut]
- Tischendorf JJ, Meier PN, Strassburg CP, et al. Characterization and clinical course of hepatobiliary carcinoma in patients with primary sclerosing cholangitis. Scand J Gastroenterol 2006;41:1227-34.[LinkOut]
- Hoffmann D, Rivenson A, Amin S, et al. Dose-response study of the carcinogenicity of tobacco-specific N-nitrosamines in F344 rats. J Cancer Res Clin Oncol 1984;108:81-6.[LinkOut]
- Murray RP, Connett JE, Zapawa LM. Does nicotine replacement therapy cause cancer? Evidence from the Lung Health Study. Nicotine Tob Res 2009;11:1076-82.[LinkOut]
- West R, Zatonski W, Cedzynska M, et al. Placebo-controlled trial of cytisine for smoking cessation. N Engl J Med 2011;365:1193-200.[LinkOut]


