Cellular and molecular mechanisms of hepatic fibrogenesis leading to liver cancer
Introduction
Currently, hepatic fibrosis is considered a model of the wound-healing response to chronic liver injury (1). The excessive extracellular matrix (ECM) deposition that distorts the hepatic architecture by forming fibrotic scars, and the subsequent development of nodules of regenerating hepatocytes defines liver cirrhosis (2-5). The clinical importance of liver cirrhosis is related to the associated hepatocellular dysfunction and increased intrahepatic resistance to blood flow, which result in hepatic insufficiency and portal hypertension, respectively, and to the occurrence of hepatocellular carcinoma (6). The incidence of hepatocellular carcinoma (HCC) is rising in North America, Europe, and Eastern countries such as China and Japan (7). This increase is largely due to the emergence of hepatitis C virus (HCV), the continued problem of hepatitis B virus (HBV) infection control, and the liver pathologies associated with obesity and chronic alcohol abuse. The increasing levels of obesity in these countries is a particularly significant epidemiological factor that will ensure further worldwide rises in HCC incidence over the next decade (8). There is therefore an urgent need to understand how HCC develops in the diseased liver. In this respect, it is often overlooked that 90% of HCC cases have a natural history of unresolved inflammation and severe fibrosis (or cirrhosis). Approaches to HCC prevention should therefore focus on the molecular regulators of a disease process that we could define as the inflammation-fibrosis-cancer (IFC) axis.
Mechanisms involved in the inflammation-fibrosis-cancer axis
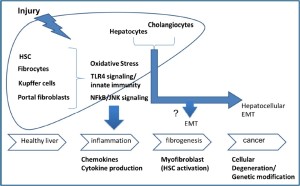
The final event of chronic liver injury, independently from the aetiological agent, is hepatic fibrosis and this process may consequently and directly lead to cancer development (Figure 1). Thus, the current review will focus the attention on the mechanisms contributing to fibrosis and cancer.
Role of oxidative stress
At the molecular level, a series of studies have shown that oxidative stress is commonly induced in all forms of chronic liver injury and plays a crucial role in hepatic fibrogenesis (9-12) and cancer development (13,14). Exogenous reactive oxygen species (ROS) released by damaged parenchymal cells directly contribute to cell degeneration process and would also activate redox-sensitive intracellular pathways in HSCs, inducing their activation and increasing collagen synthesis (11,15). Furthermore, HSCs are also an important source of ROS in liver fibrosis (15-17). Cytochrome P450 2E1 is the main source of ROS in hepatocytes, while phagocytic and non-phagocytic nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is the key source, respectively, in Kupffer cells and HSCs (12,18,19). The phagocytic form of NADPH oxidase expressed in Kupffer cells has several important functions: besides its defensive effect against bacterial products reaching the liver through the portal system, NADPH oxidase in Kupffer cells is also activated by several stimuli (i.e. alcohol metabolites and tumor necrosis factor-α) to produce ROS. Kupffer cellsderived ROS consequently drive proinflammatory effects and sensitize hepatocytes to undergo apoptosis, being involved in fibrogenesis and carcinogenesis. Conversely, recent data indicate that HSCs express the non-phagocytic form of NADPH oxidase and demonstrate that ROS participate in the activation and fibrogenic actions of HSCs in vitro (12,18,19). Thus in summary, several sources of ROS in parenchymal and non-parenchymal cells actively contribute to the development and activation of pathways involved either in fibrogenic or in cancer processes.
Role of cytokines
Classical studies using experimental models of chronic liver injury in rats and mice have revealed cytokines and growth factors that are critical in hepatic fibrogenesis (20-22) as well as in cancer development (1,13). As occurs in most tissues, transforming growth factor-b1 (TGF-β1) is the major fibrogenic cytokine in the liver (23) and it has been clearly demonstrated to play an active role in the process of myofibroblast activation (1). In addition to TGF-β1, other molecules exert a pro-fibrogenic activity involving different mechanisms: vasoconstrictor substances [e.g., norepinephrine (NE), angiotensin II] (24), platelet-derived growth factor (PDGF) (the most potent mitogen) (25) and adipocytokines such as leptin involved in hepatic inflammation (26,27). As in hepatic fibrosis, TGF-β levels in HCC increase in line with collagen deposition and the reduction in proteolytic degradation (23). For example, connective tissue growth factor (CTGF) promotes tumor growth, angiogenesis, migration and invasion (28). Human HCC cell lines produce high levels of CTGF to form highly stromogenic tumors. If CTGF is knocked down in such cells, tumors show little stroma (29). Blocking the TGF-β signaling pathway with the TGF-β inhibitor LY2109761 inhibits CTGF production and tumor growth. Based on these observations, cancer associated fibroblasts (CAF) are considered a possible source of CTGF in response to paracrine signals from cancer cells, such as TGF-β (30). It appears that CAF can originate from endothelial cells and can be a source of the endothelial-to-mesenchymal transformation (30). Also, in pancreatic cancer they can originate from stellate cells and can contribute to resistance to chemotherapy or radiation (31). Thus, it is possible that HSC may be the source for the CAF cells in HCC.
Role of NFkB
NF-κB transcription factors are key regulators of innate and adaptive immune responses, inflammation, and cell survival (32,33). Many proinflammatory stimuli activate NF-κB, mainly via IκB kinase-(IKK) dependent phosphorylation and degradation of the κB inhibitor (IκB) proteins. IKK consists of two catalytic subunits, IKKα and IKKβ, and a regulatory component, NEMO/IKKγ. IKK activation occurs primarily through IKKβ (34), whose absence increases susceptibility to tumor necrosis factor-α-(TNFα-) induced apoptosis (35). Tumor initiation means cellular immortality, which happens through DNA mutation, but the relationship with NF-κB activation has not been considered in detail for this process. However, the first clue linking NF-κB to cancer was recognizing that c-rel, which is a v-rel oncogene cellular homologue, encodes an NF-κB subunit and that all of these proteins share the Rel homology DNA-binding domain (36). Not surprisingly, overexpression of normal Rel proteins promotes oncogenic transformation. Participation of NF-κB activation in the carcinogenic promotion and progression stages has become clear in recent years. The promotion of carcinogenesis is mainly related to the involvement of NFkB in the regulation of different processes such as: proliferation, apoptosis, angiogenesis, invasion, and metastasis (36,37). TNFα, which is a strong NF- κB-activating factor, is produced by macrophages and plays a central role in inflammation but has also been suggested as an accelerator factor of cell proliferation (13,37). Anti-apoptosis is also important for maintaining cancer cells: a large number of antiapoptotic factors, such as cIAPs, c-FLIP, and BclX, are controlled by NF-κB activation (38). Finally, invasion and metastasis are pivotal processes for prognosis: matrix metalloproteinases (MMPs) are produced by inflammatory cells and on the other hand tumor cells are key players in the degradation of the extracellular matrix and basement membranes; thus, they are important in tumor invasion.
Role of JNK
The c-Jun NH2-terminal kinase (JNK) belongs to a family of mitogen-activated kinases (MAPKs), together with extracellular regulated kinases (ERKs) and p38. The JNK subgroup of MAPKs is encoded by three loci; Jnk1 and Jnk2 are ubiquitously expressed, and Jnk3 is expressed primarily in heart, testis, and brain (39). JNKs are activated by stress signals and proinflammatory stimuli, and their activity increases following phosphorylation by the MAPK kinases, MKK4, and MKK7 (40). In the liver, JNK plays a pivotal role in the development of metabolic syndrome including NAFLD (20). Hepatic steatosis, inflammation, and fibrosis have been examined in mice fed a choline-deficient L-amino-acid-defined diet. The results showed less hepatic inflammation and less liver fibrosis despite a similar level of hepatic steatosis in JNK1-deficient mice compared with wild type, suggesting that JNK1 may be associated with the induction of diet-induced steatohepatitis and liver fibrosis (41). In addition to these data, JNK function is critical in the carcinogenic promotion and progression stages, as JNK phosphorylates a variety of genes associated with carcinogenesis. Growth factors activate receptor tyrosine kinases, and phosphorylated receptors transmit the signals through JNKs (42). There is also participation in the transcriptional regulation of growth factors such as EGF through JNK activation (24). Numerous studies have considered the proliferative effect following JNK activation. For example, in a liver regeneration mouse model, the number of Ki67-positive proliferating hepatocytes in Jnk1-/- mice was reduced by 80% compared with that in controls at 48 hours after a partial hepatectomy (43). The expression of several angiogenic factors is also regulated by JNK: Vascular endothelial growth factor (VEGF) promotes proliferation and migration of endothelium cells. VEGF expression is also controlled by JNK activation (44,45).
Role of TLRs
Innate immunity represents the first line of protection against microbial pathogens and is mediated by macrophages and dendritic cells. Although it was initially suggested to be a nonspecific response, innate immunity discriminates a variety of pathogens through the function of pattern-recognition receptors (PRRs) such as TLRs. These receptors recognize microbial components known as pathogen-associated molecular patterns (41,46). Thirteen mammalian TLRs have been described; 10 are expressed in humans, and each is responsible for recognizing distinct bacteria, virus, and fungi microbial structures: the two most largely studied are TLR2 and TLR4, the PRRs for gram-negative and gram-positive bacterial products, respectively. TLR4 is also the major receptor recognizing endogenous ligands released from damaged or dying cells (41,42). The liver may be exposed to bacteria from the intestine via the portal vein, leading to an uncontrolled innate immune system that may result in inflammatory liver disorders (47). Many factors are capable of activating TLRs in the liver. Among them, HBV, HCV, alcoholic liver disease, and NASH are important etiologies for HCC (48). The TLR ligands TLR4 and 9 inhibit viral replication in HBV-transgenic mice (49). In the absence of HBeAg, HBV replication is associated with upregulation of the TLR2 pathway, leading to increased TNFα production, demonstrating a potentially important interaction between HBV and the innate immune response (42,50). HCV can activate innate immune systems to produce inflammation. The HCV core and NS3 proteins activate TLR2 on monocytes to induce cytokines in a NF-κBand JNK-dependent manner (51). The NS3 protein interacts directly with TBK1, resulting in decreased TBK1-IRF3 interaction and inhibition of IRF3 and IFN transcription. The NS3 protein also impedes both IRF3 and NF-κB activation by reducing functional TRIF abundance (52). Many other in vitro studies have been reported, but the in vivo condition is still unclear. Excessive alcohol intake is associated with increased intestinal permeability and elevated endotoxin levels (50). LPS activates TLR4 on Kupffer cells and increases proinflammatory cytokine production. Antibiotic treatment reduces the sensitivity of alcoholic liver disease (53). Intestinal bacteria seem to be important in NASH pathogenesis. In NASH, ob/ob mice exhibit increased hepatic sensitivity to LPS and developed steatohepatitis (54). In a methionine/choline-deficient NASH model, TLR4-deficient, but not TLR2-deficient mice, exhibited less intrahepatic lipid accumulation (53). All of the diseases described above are associated with the development of HCC. Therefore, it seems clear that TLRs are involved in the development of HCC. Mice deficient in TLR4 and MyD88, but not TLR2, have a marked decrease in the incidence, size, and number of chemically induced (DEN) liver cancer tumors, indicating a strong contribution of TLR signaling to hepatocarcinogenesis (55). It is assumed that dying hepatocytes following DEN may activate myeloid cells such as Kupffer cells via TLRs and induce proinflammatory cytokines and hepatomitogens, which enhance the development of HCC.
Role of EMT
Epithelial to Mesenchmal Transition (EMT) may also occur in the liver. Fetal liver exhibits characteristics of EMT in that some fibroblast-like stromal cells co-express both epithelial (a-fetoprotein [AFP], albumin [Alb], cytokeratins CK18 and CK7) and mesenchymal markers (a-SMA, osteopontin, and collagen I) (56,57). In adult liver, EMT does not occurs without stress or injury (56). Transformation of biliary epithelium into fibroblasts is best documented in metastatic hepatocellular carcinoma, while is more controversial in the field of liver fibrosis. Evidence of EMT in liver fibrosis was reported in a patient with primary biliary cirrhosis (PBC), a condition characterized by loss of biliary epithelial cells and progressive fibrosis (58). Analysis of liver biopsies from this patient revealed that a number of biliary epithelial cells expressed markers of EMT (e.g., an early fibroblast marker FSP1, vimentin, nuclear Smad2/3 and a-SMA), suggesting that biliary epithelial cells may undergo EMT and potentially contribute to the fibroblast population (58). In mice, EMT was observed in response to bile duct ligation (BDL)-induced injury (59). BDL causes chronic obstruction and concomitant proliferation of the bile duct, outgrowth of periductal myofibroblasts and fibrosis. Expression of EMT markers (collagen type I, a-SMA, and cytokeratin 19) by periductal myofibroblasts supported a notion that biliary epithelial cells may undergo EMT (59).
On the other hand, scientifically relevant data demonstrate the absence of EMT in liver fibrosis: the report by the Wells laboratory provides the strongest evidence against EMT in the liver as a source of myofibroblasts (60). The study uses lineage tracing generated by crossing the alpha-fetoprotein (AFP)-cre mouse with the ROSA26YFP stop mouse to trace the fate of any cell ever expressing AFP. As expected, all the cholangiocytes and all the hepatocytes were genetically labeled, because they are derived from AFP-expressing precursor cells. Furthermore, AFP progenitor cells were also irreversibly genetically marked. The critical result is that after inducing liver fibrosis by a variety of methods, none of the resulting myofibroblasts originated from the genetically marked epithelial (AFP) cells. This important article confirm the results obtained in two previous studies demonstrating the contribution of epithelial cells to myofibroblasts in liver fibrosis (61,62).
While EMT in hepatic fibrosis play a controversial role, the importance of this process in the development of HCC is relevant and is known as hepatocellular EMT. Hepatocellular EMT has been recognized not only in experimental animal model but also in humans and it is mainly defined as the expression of epithelial markers in cancer cells. In fact, while hepatocytes of well-differentiated human HCC samples and adjacent non-cancerous liver parenchyma show E-cadherin at the plasma membranes, cytoplasmic localization or frequent loss of E-cadherin is displayed in poorly differentiated HCC. These data suggest a disruption of E-cadherin/β-catenin complexes at cell boundaries that is characteristic for hepatocellular EMT and comparable to observation of experimental HCC in mouse (63). The reduced expression of E-cadherin is accompanied by (partial) nuclear translocation of β-catenin, and significantly correlates with intrahepatic metastasis and poor survival of patients.
Experimental models of liver fibrosis/cancer and future directions
Although the number of studies on the field of liver fibrosis and HCC degeneration, there is an important need of a model that could be used in basic research, able to resemble the human characteristics of HCC development starting from a condition of liver disease, as it happens in humans. The models existing in literature are not completely accurate and do not entirely recreate the human conditions: the limitations are mainly related to the lack of a tool that could summarize all the features in a single experimental model.
Concerning experimental models of HCC: genetic models, conditioned knock-out or transgenic models are mainly used to study the involvement of specific protein in the carcinogenetic process (64,65). On the other hand, the HCC models induced by chemotoxic agents (such as DEN model) allow a broader involvement of different pathways, providing a technique to study the interaction of different effects in the specific organ. However, chemotoxic-induced HCC models do not completely resemble the human disease.
The DEN is the most important and most used agent in literature. Such a compound allows to obtain HCC development in a time and dose dependent manner, being also easy to reproduce. The DEN model has been largely used to study the pathophysiology of the pure HCC tumor model (66,67). However, in this model the sequence of events leading to steatohepatitis, fibrosis, cirrhosis and tumor, is completely skipped. Based on this, recent manuscripts evaluated other models able to better recreate the conditions leading to HCC development in cirrhotic patients (68). In the current view, the diet model could be a good option, allowing researchers to study not only the mechanisms involved in tumor progression, but also the early events involved in tumor formation.
References
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209-18.[LinkOut]
- Bonis PA, Friedman SL, Kaplan MM. Is liver fibrosis reversible? N Engl J Med 2001;344:452-4.[LinkOut]
- Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol 2003;38:S38-53.[LinkOut]
- Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 2008;134:1655-69.[LinkOut]
- Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol 2011;6:425-56.[LinkOut]
- Wong CM, Wong CC, Lee JM, et al. Sequential alterations of miRNA expression in hepatocellular carcinoma development and venous metastasis. Hepatology 2011.[LinkOut]
- Papatheodoridis GV, Lampertico P, Manolakopoulos S, et al. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol 2010;53:348-56.[LinkOut]
- Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010;51:1820-32.[LinkOut]
- Marra F, Gastaldelli A, Svegliati Baroni G, et al. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med 2008;14:72-81.[LinkOut]
- Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol 2007;22:S73-8.[LinkOut]
- Brenner DA, Seki E, Taura K, et al. Non-alcoholic steatohepatitis-induced fibrosis: Toll-like receptors, reactive oxygen species and Jun N-terminal kinase. Hepatol Res 2011;41:683-6.[LinkOut]
- De Minicis S, Brenner DA. Oxidative stress in alcoholic liver disease: role of NADPH oxidase complex. J Gastroenterol Hepatol 2008;23:S98-103.[LinkOut]
- Aoyama T, Inokuchi S, Brenner DA, et al. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology 2010;52:1390-400.[LinkOut]
- Tanaka S, Mogushi K, Yasen M, et al. Oxidative stress pathways in noncancerous human liver tissue to predict hepatocellular carcinoma recurrence: a prospective, multicenter study. Hepatology 2011;54:1273-81.[LinkOut]
- De Minicis S, Seki E, Paik YH, et al. Role and cellular source of nicotinamide adenine dinucleotide phosphate oxidase in hepatic fibrosis. Hepatology 2010;52:1420-30.[LinkOut]
- Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact 2011;193:225-31.[LinkOut]
- Paik YH, Iwaisako K, Seki E, et al. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology 2011;53:1730-41.[LinkOut]
- Marzioni M, Saccomanno S, Candelaresi C, et al. Clinical implications of novel aspects of biliary pathophysiology. Dig Liver Dis 2010;42:238-44.[LinkOut]
- Svegliati-Baroni G, De Minicis S, Marzioni M. Hepatic fibrogenesis in response to chronic liver injury: novel insights on the role of cell-to-cell interaction and transition. Liver Int 2008;28:1052-64.[LinkOut]
- Kodama Y, Kisseleva T, Iwaisako K, et al. c-Jun N-terminal kinase-1 from hematopoietic cells mediates progression from hepatic steatosis to steatohepatitis and fibrosis in mice. Gastroenterology 2009;137:1467- 1477.e5.[LinkOut]
- Lin W, Tsai WL, Shao RX, et al. Hepatitis C virus regulates transforming growth factor beta1 production through the generation of reactive oxygen species in a nuclear factor kappaB-dependent manner. Gastroenterology 2010;138:2509-18, 2518.e1.[LinkOut]
- Marra F, Bertolani C. Adipokines in liver diseases. Hepatology 2009;50:957-69.[LinkOut]
- Brenner DA. Molecular pathogenesis of liver fibrosis. Trans Am Clin Climatol Assoc 2009;120:361-8.[LinkOut]
- Bataller R, Schwabe RF, Choi YH, et al. NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest 2003;112:1383-94.[LinkOut]
- Pinzani M, Gesualdo L, Sabbah GM, et al. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest 1989;84:1786-93.[LinkOut]
- Wang J, Leclercq I, Brymora JM, et al. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology 2009;137:713-23.[LinkOut]
- Elinav E, Ali M, Bruck R, et al. Competitive inhibition of leptin signaling results in amelioration of liver fibrosis through modulation of stellate cell function. Hepatology 2009;49:278-86.[LinkOut]
- Liu J, Ding X, Tang J, et al. Enhancement of Canonical Wnt/β-Catenin Signaling Activity by HCV Core Protein Promotes Cell Growth of Hepatocellular Carcinoma Cells. PLoS One 2011;6:e27496.[LinkOut]
- Meurer SK, Esser M, Tihaa L, et al. BMP-7/TGF-β1 signalling in myoblasts: Components involved in signalling and BMP-7-dependent blockage of TGF-β-mediated CTGF expression. Eur J Cell Biol 2011. [Epub ahead of print].[LinkOut]
- Wang B, Haldar SM, Lu Y, et al. The Kruppel-like factor KLF15 inhibits connective tissue growth factor (CTGF) expression in cardiac fibroblasts. J Mol Cell Cardiol 2008;45:193-7.[LinkOut]
- Walter K, Omura N, Hong SM, et al. Overexpression of smoothened activates the sonic hedgehog signaling pathway in pancreatic cancerassociated fibroblasts. Clin Cancer Res 2010;16:1781-9.[LinkOut]
- Saile B, Matthes N, El Armouche H, et al. The bcl, NFkappaB and p53/ p21WAF1 systems are involved in spontaneous apoptosis and in the antiapoptotic effect of TGF-beta or TNF-alpha on activated hepatic stellate cells. Eur J Cell Biol 2001;80:554-61.[LinkOut]
- Kühnel F, Zender L, Paul Y, et al. NFkappaB mediates apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. J Biol Chem 2000;275:6421-7.[LinkOut]
- Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alphainduced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol 2006;290:G583-9.[LinkOut]
- Russo MP, Schwabe RF, Sartor RB, et al. NF-kappaB-inducing kinase restores defective IkappaB kinase activity and NF-kappaB signaling in intestinal epithelial cells. Cell Signal 2004;16:741-50.[LinkOut]
- Factor P. Role and regulation of lung Na,K-ATPase. Cell Mol Biol (Noisyle- grand) 2001;47:347-61.[LinkOut]
- Calvisi DF, Pascale RM, Feo F. Dissection of signal transduction pathways as a tool for the development of targeted therapies of hepatocellular carcinoma. Rev Recent Clin Trials 2007;2:217-36.[LinkOut]
- Kaisho T, Takeda K, Tsujimura T, et al. IkappaB kinase alpha is essential for mature B cell development and function. J Exp Med 2001;193:417-26.[LinkOut]
- Jablonska E, Markart P, Zakrzewicz D, et al. Transforming growth factor-β1 induces expression of human coagulation factor XII via Smad3 and JNK signaling pathways in human lung fibroblasts. J Biol Chem 2010;285:11638-51.[LinkOut]
- Hanczko R , Fernandez DR , Doher t y E, et al . Prevent ion of hepatocarcinogenesis and increased susceptibility to acetaminopheninduced liver failure in transaldolase-deficient mice by N-acetylcysteine. J Clin Invest 2009;119:1546-57.[LinkOut]
- Kluwe J, Pradere JP, Gwak GY, et al. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology 2010;138:347-59.[LinkOut]
- Matsuzaki K, Murata M, Yoshida K, et al. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology 2007;46:48-57.[LinkOut]
- Thevananther S, Sun H, Li D, et al. Extracellular ATP activates c-jun N-terminal kinase signaling and cell cycle progression in hepatocytes. Hepatology 2004;39:393-402.[LinkOut]
- Kanematsu M, Osada S, Amaoka N, et al. Expression of vascular endothelial growth factor in hepatocellular carcinoma and the surrounding liver: correlation with MR imaging and angiographically assisted CT. Abdom Imaging 2006;31:78-89.[LinkOut]
- Limaye PB, Bowen WC, Orr AV, et al. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology 2008;47:1702-13.[LinkOut]
- Inokuchi S, Aoyama T, Miura K, et al. Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc Natl Acad Sci U S A 2010;107:844-9.[LinkOut]
- Roth CL, Elfers CT, Figlewicz DP, et al. Vitamin D deficiency in obese rats exacerbates NAFLD and increases hepatic resistin and toll-like receptor activation. Hepatology 2011. [Epub ahead of print].[LinkOut]
- Sène D, Levasseur F, Abel M, et al. Hepatitis C virus (HCV) evades NKG2D-dependent NK cell responses through NS5A-mediated imbalance of inflammatory cytokines. PLoS Pathog 2010;6:e1001184.[LinkOut]
- Wu J, Lu M, Meng Z, et al. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology 2007;46:1769-78.[LinkOut]
- Szabo G, Bala S. Alcoholic liver disease and the gut-liver axis. World J Gastroenterol 2010;16:1321-9.[LinkOut]
- Machida K, Tsukamoto H, Mkrtchyan H, et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci U S A 2009;106:1548-53.[LinkOut]
- Zhao XJ, Dong Q, Bindas J, et al. TRIF and IRF-3 binding to the TNF promoter results in macrophage TNF dysregulation and steatosis induced by chronic ethanol. J Immunol 2008;181:3049-56.[LinkOut]
- Csak T, Velayudham A, Hritz I, et al. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol 2011;300:G433-41.[LinkOut]
- Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol 2009;51:212-23.[LinkOut]
- Yu LX, Yan HX, Liu Q, et al. Endotoxin accumulation prevents carcinogeninduced apoptosis and promotes liver tumorigenesis in rodents. Hepatology 2010;52:1322-33.[LinkOut]
- Milani S, Herbst H, Schuppan D, et al. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol 1991;139:1221-9.[LinkOut]
- Sedlaczek N, Jia JD, Bauer M, et al. Proliferating bile duct epithelial cells are a major source of connective tissue growth factor in rat biliary fibrosis. Am J Pathol 2001;158:1239-44.[LinkOut]
- Hsieh CS, Huang CC, Wu JJ, et al. Ascending cholangitis provokes IL-8 and MCP-1 expression and promotes inflammatory cell infiltration in the cholestatic rat liver. J Pediatr Surg 2001;36:1623-8.[LinkOut]
- Benyon RC, Iredale JP, Goddard S, et al. Expression of tissue inhibitor of metalloproteinases 1 and 2 is increased in fibrotic human liver. Gastroenterology 1996;110:821-31.[LinkOut]
- Chu AS, Diaz R, Hui JJ, et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology 2011;53:1685-95.[LinkOut]
- Taura K, Miura K, Iwaisako K, et al. Hepatocytes do not undergo epithelialmesenchymal transition in liver fibrosis in mice. Hepatology 2010;51:1027-36.[LinkOut]
- Scholten D, Osterreicher CH, Scholten A, et al. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology 2010;139:987-98.[LinkOut]
- Zhai B, Yan HX, Liu SQ, et al. Reduced expression of E-cadherin/ catenin complex in hepatocellular carcinomas. World J Gastroenterol 2008;14:5665-73.[LinkOut]
- Feng H, Cheng AS, Tsang DP, et al. Cell cycle-related kinase is a direct androgen receptor-regulated gene that drives β-catenin/T cell factordependent hepatocarcinogenesis. J Clin Invest 2011;121:3159-75.[LinkOut]
- Koch KS, Maeda S, He G, et al. Targeted deletion of hepatocyte Ikkbeta confers growth advantages. Biochem Biophys Res Commun 2009;380:349- 54.[LinkOut]
- Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007;317:121-4.[LinkOut]
- Maeda S, Kamata H, Luo JL, et al. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 2005;121:977-90.[LinkOut]
- de Lima VM, Oliveira CP, Alves VA, et al. A rodent model of NASH with cirrhosis, oval cell proliferation and hepatocellular carcinoma. J Hepatol 2008;49:1055-61.[LinkOut]