TNF-α in obesity-associated colon cancer
Introduction
Modern societies are challenged by dramatic changes in the epidemiology of diseases. Scientific and technological advances have resulted in more efficient treatment of acute diseases and changes in human habits contributing to a high increase in the prevalence of chronic inflammatory conditions. In this context, obesity and cancer have emerged as two of the greatest threats to global human health. Here we will examine the evidence that links inflammation as a key mechanism to promote both obesity and cancer. We will extend the discussion to present the pathophysiological mechanisms that implicate obesity-associated inflammation in the development of colorectal cancer, with a special emphasis on the role of TNF-α.
Inflammation: basis for modern diseases
Inflammation is canonically defined as an essential biological response which promotes host repairs of tissue injury and infection (1). In the last decades, striking advances were made in our understanding of the biochemical and cellular mechanisms induced by acute inflammation, whilst the knowledge of the intracellular programs regulated by chronic inflammation advanced at a much slower rate (2). Nevertheless, the spectrum of prevailing inflammatory conditions has shifted from acute to chronic inflammatory states since the end of 20th century, significantly contributing to the pathogenesis of modern diseases such as obesity, type 2 diabetes (3,4), atherosclerosis (5), neurodegenerative diseases (6), and certain cancers (7).
The most obvious signs of inflammation are heat, pain, swelling, and redness, described by Celsius during the time of the Roman Empire. Initially, this inflammatory response was deemed as a biological reaction without deleterious effects, evoked just to protect from infection and normalize homeostasis. This theory influenced the understanding of the field until the 1970s, when it was recognized that inflammation not only preserves the integrity of the body but might also harm host tissues itself (8). Interestingly, recent research brought to light the fact that inflammation-mediated deleterious effects are closely linked to the pathophysiology of chronic multifactorial diseases (9-12). Accordingly, there is increasing interest in the mechanisms involved in the resolution of inflammatory response as much evidence links nonresolving inflammation to the pathophysiology of the ever-growing modern diseases of industrialized societies (10).
There is intense debate about the regulatory mechanisms that control inflammatory response, in part due to its complexity and also because of the multitude of agents involved in its induction and resolution. However, it is now well recognized that there are two major stimuli that promote acute inflammation: infection and host cell necrosis from sterile tissue injury (13). Intriguingly, the products generated by both processes are recognizable by the same cluster of host molecules, which activate a common inflammatory pathway that eliminates triggering stimuli and repairs the damaged tissue (2). As a result, inflammation is often interrupted by an active and highly regulated process that restores the homeostatic state (2,14,15). One key regulating mechanism of inflammation resolution is the switch from pro-inflammatory prostaglandins and leukotrienes to anti-inflammatory resolution-inducing lipids, such as lipoxins and resolvins (14,16). Specifically, these anti-inflammatory mediators promote the transition from neutrophil to monocyte recruitment (17-19). The subsequent uptake of apoptotic neutrophils orchestrates the production of anti-inflammatory cytokines by monocytes and recruited macrophages, which are responsible for the clearance of dead cells and other debris and initiation of tissue repair at the damaged site (15,20,21). However, if the inflammatory trigger is not eliminated, a chronic state of inflammation is sustained for an undetermined period of time, although signs of acute phase may reappear throughout the course of the disease. This type of chronic inflammation is detected in a myriad of conditions including tuberculosis, unrepaired tissue damage, persistent allergens and undigestible foreign particles and endogenous crystals (10).
Chronic inflammation may also occur in diseases where the initiating trigger is not well defined and does not seem to be related to infection or tissue damage, therefore, without a physiological counterpart (2,9). In these conditions, inflammation appears to be chronic from the outset with infiltration of monocytes, dendritic cells and macrophages into the target tissue. Examples include obesity (22), atherosclerosis (5) and some cancers (23). Notably, in these cases of chronic inflammation there appears to be vicious cycles connecting inflammation and the pathological process it accompanies. Indeed, this reciprocal relationship may be responsible, at least in part, for the chronic nature of these inflammatory conditions and distinguishes them from the first type of chronic inflammation, which is caused by the persistence of the inflammatory inducer.
A causal relationship between chronic inflammation and cancer has long been suspected. It was first detected by Galen and later established in the 19th century by Rudolf Virchow who discovered leukocyte infiltration in malignant tissues. Interestingly, the inflammatory response is similar in many aspects to a wound-healing process and tumors have been considered as wounds that do not heal (24). Research over the last decade in the field of inflammation and cancer pathogenesis has produced abundant evidence of the functionally important tumor-promoting effects that immune cell have on neoplastic progression (7,23,25). Inflammation can contribute to multiple hallmark capabilities by supplying bioactive molecules to the tumor microenvironment, including growth factors that sustain proliferative signaling, survival factors that limit cell death, proangiogenic factors, extracellular matrix-modifying enzymes while enhancing cell proliferation, cell survival, cell migration and angiogenesis (7,23,25). Accordingly, the importance of inflammation for production of the “tumor microenvironment” is now widely recognized as an enabling characteristic of cancer (26).
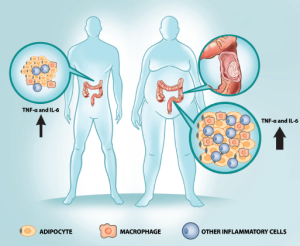
As a modern epidemic disease, the concept of obesity-induced adipose tissue inflammation is much more recent, about 20 years old (27). Corresponding to Virchow’s findings related to cancer tissue, large numbers of macrophages have been observed infiltrating adipose tissue from obese mice and humans (28,29). In obesity, the proinflammatory pathways in adipose tissue macrophages (ATM) are highly activated, leading to the secretion of a variety of cytokines such as TNF-α and interleukin-6 (IL-6) (3,30).
Inflammation is conspicuously associated with certain colon cancers. For instance, colitis-associated cancer (CAC) often arises in patients diagnosed with inflammatory bowel disease (IBD) including Crohn’s disease and ulcerative colitis (31). Moreover, the cumulative incidence of CAC among patients with ulcerative colitis 25 years after diagnosis ranges from 8% to 32%, accounting for one sixth of all deaths in this group (31). Furthermore, Crohn’s disease is associated with a pooled estimated relative risk of 2.4 (32). The physiopathology of IBD is multifactorial and involves genetic, mucosal, microbiota and immune system abnormalities (for review see Xavier et al. and Danese et al.) (33,34). Interestingly, the disrupted communication between the epithelium and the intestinal flora has an important role in activating the immune system and maintaining the inflammatory response (35-40). Therefore, ulcerative colitis and CAC are mainly mediated by the first mentioned mechanism of nonresolving inflammation, whereby the inflammatory trigger is not eliminated and causes an acute inflammatory response to persist for a long period of time.
In addition to IBD, other well-known risk factors for colon cancer are obesity, diets low in fruits and vegetables, and physical inactivity (41,42). As these habits were initially more prevalent in developed nations, obesity-associated cancer was once a disease primarily observed in longstanding industrialized societies; however nowadays it is a worldwide health burden (43). Specifically, the association between being overweight or obese with colon cancer are positive for both men (RR =1.24) and women (RR =1.09) at an elevation of 5 kg/m2 in BMI (42). Intriguingly, obesity-associated colon cancer is, at least in part, mediated by the second mentioned mechanism of nonresolving inflammation, in which chronic low-grade inflammation arises without a clear trigger. In the next topics we will further explore these inflammatory features of obesity-associated colon cancer.
Obesity-associated inflammation
In the 1980s and 1990s, the world saw a striking increase in the prevalence of obesity and in the most recent years it trended to levelling out (44). This epidemic had begun in developed countries, but nowadays it is also common in many other regions over the world, such as Asia and Latin America (43,45-47). In conjunction with this epidemic, we faced a dramatic increase in the prevalence of diseases, such as hypertension, dyslipidemia, cardiovascular disease, type 2 diabetes mellitus and certain cancers, making obesity a worldwide public health concern (48).
Obesity-associated tissue inflammation is now recognized as a major driver in the pathogenesis of metabolic diseases (3,4,49,50). Activation of inflammatory pathways has since been observed in classical metabolic tissues, including fat, liver and muscle (27,51,52). At the molecular level, chronic low-grade inflammation induced by obesity leads to activation of protein kinases, such as Jun N-terminal kinase (JNKs) (53) and inhibitor of nuclear factor B kinaseβ (IKKβ) (51,54,55), which phosphorylates serine 307 (Ser307) of IRS-1 (56,57). As a result, the interaction of the PTB domain of IR with the phosphorylated NPEY motif of IRS-1 is inhibited, impairing the interaction of IRS-1 with the insulin receptor and causing insulin resistance (56). Obesity associated inflammation is also associated with increased activity of iNOS, which S-nitrosates insulin signaling pathway and promotes insulin resistance (58-61).
A pivotal event in the pathophysiology of obesity-induced inflammation is the recruitment of macrophages into adipose tissue (62). The large accumulation of adipose tissue macrophages (ATMs), representing up to 40% of the cells in obese adipose tissue, determines locally increased levels of pro-inflammatory cytokines, such as TNF-α and IL-6, which sustain insulin resistance in a paracrine manner (28,29,55). In addition, these cytokines may also leak out the adipose tissue and exert systemic effects (28). Congruent with this data, macrophages are recruited to adipose tissue by chemokines secreted by adipocytes, which provide a chemotactic gradient that attracts Ly6Chi monocytes into the adipose tissue, where they differentiate into ATMs (63-66). Once pro-inflammatory ATMs migrate into adipose tissue, they also secrete their own chemokines, attracting additional macrophages and establishing a vicious cycle that stimulates the inflammatory process (55).
Macrophages are dynamic cells that acquire different phenotypes in accordance with the microenvironment that they reside (62). These cells are often classified by their functional inflammatory state and the polarized states are often referred to as classically activated macrophages (CAMs), known as M1, and alternatively activated macrophages (AAMs), known as M2 (67). In adipose tissue these two subpopulations exert opposite immune actions: M1 inflammatory macrophages secrete proinflammatory cytokines whereas AAMs secrete anti-inflammatory ones (22). The majority of ATMs in obesity are M1-like, identified by the specific expression of CD11c, typically negative in M2-like macrophages that reside in lean adipose tissue (55,68). Along this line, macrophage specific JNK deficient mice are protected from insulin resistance induced by high fat diet (69). In contrast, repression of programs that control alternative activation of macrophages is associated with obesity and insulin resistance (70,71). Furthermore, obese animals exposed to a switch from a high-fat diet (HFD) to a chow diet or treated with omega-3-fatty acids or thiazolinediones have macrophages converted from an M1 to M2 phenotype, coincident with increased insulin sensitivity (72,73).
After the observation of the striking switch from AAM to inflammatory macrophages in obese adipose tissue, it was progressively described that not only are macrophages actively mobilized by the obese adipose tissue but also by other innate and adaptive immune cells (22,74). In a simplified way, there is an increase in inflammatory immune cells such as Th1 cells (75), CD8+ T cells (76) and B cells (77), which promote insulin resistance by further activating inflammatory macrophages or directly secreting pro-inflammatory cytokines or antibodies. Meanwhile, this pool of inflammatory cells takes place with resident tolerogenic immune cells, including eosinophils (68), innate lymphoid type 2 cells (ILC2s) (78), regulatory T cells (Tregs) (79), invariant natural killer (iNKT) cells (80,81) and Th2 cells (75), which secrete IL-4, IL-5 and/or IL-10 and, therefore, promote direct anti-inflammatory effects or activate the alternative program of resident macrophages to sustain metabolic homeostasis. Despite the debate about the sequence of cells that infiltrate the adipose tissue, obesity assembles a large number of immune cells that promote and amplify the inflammatory response in the adipose tissue.
Another critical mechanism that mediates inflammation in obesity is the interaction with the host-microbiota (82,83). The gut microbiota contains expressive amounts of lipopolysaccharides (LPS) derived from Gram-negative bacteria, which can leak into circulation and may cause inflammation and macrophage recruitment into adipose tissue (84,85). Interestingly, recent studies revealed that obesity changes in microbiota are associated with increased circulating LPS levels (86,87). Accordingly, exercise-induced decreases in LPS circulating levels parallels the increase in insulin sensitivity (88). Mechanistically, LPS binds to TLR-4 Toll-like receptors (TLRs), which exert a central role as a major regulator in microbe-associated molecules recognition and free fatty acids (89). Importantly, TLR4 activation promotes increased JNK and IKK activity and insulin resistance in obesity (76). In addition, TLR4 genetically deficient animals were protected from free fatty acids- and obesity-induced insulin resistance (89,90). Interestingly, gut microbiota modulation by antibiotic treatment decreases LPS and TLR4 activation sustains insulin sensitivity in different animal models (85,87,91).
In aggregate, the studies discussed in this section suggest that obesity is a unique systemic chronic inflammatory disease. Importantly, the interplay between cytokines secreted by inflammatory cells, free fatty acids and gut microbiota products signal through the two prototypical pro-inflammatory receptors, TNF-α and TLR-4 promoting the activation of specific intracellular cascades that include IKK-β, NF-κB and JNK and resulting in the inhibition of insulin signaling and deregulation of metabolic homeostasis. Interestingly, insulin resistance has been suggested to be an adaptive and protective response that properly balances the metabolic homeostasis during the noxious stimulus of overnutrition (3). Since the protective effects of inflammation cannot be dissociated from a cost to homeostasis (92), it is important to better understand how obesity-associated inflammation also promotes human modern diseases including cancer (Figure 1).
Obesity-associated inflammation and colon cancer
Besides type 2 diabetes, hyperlipidemia and hypertension, which are classically linked to obesity, other diseases, including cancer, were recently associated with obesity (93). Obesity not only promotes colorectal cancer (CRC) but it is also specifically associated with esophageal, pancreatic, post-menopausal breast, endometrial, thyroid, gallbladder and renal cancers (42). Notably, a meta-analysis of 56 studies, where more than 7 million individuals were evaluated, demonstrated that for each 5 kg/m2 increment in body mass index BMI there was an increase of 18% in the risk of developing colon cancer (94).
In spite of the prominent epidemiological importance of obesity as a risk factor for colon cancer, the initial evidence that implicates inflammation as a promoter of colon cancer comes from CAC studies. Remarkably, TNF-α production is increased in ulcerative colitis and has been implicated in its pathogenesis (95,96). Although, it is long recognized that TNF-α activates the oncogenic transcription factors NF-κB and AP-1 only recently the importance of inflammatory cytokines in CAC became better understood (97,98). In an elegant study by Greten et al., the conditional ablation of IKKβ in epithelial cells resulted in a marked reduction in the development of colonic adenomas, but had little effect on adenoma size (99). Otherwise, lack of NF-κB in myeloid cells, principally lamina propria macrophages, led to a significant reduction in both colonic tumor quantity and size (99). Although IKKβ ablation did not resulted in decreased TNF-α production, it is not clear whether the LysM-Cre deleter used in this study is non-functional in a specific subset of colonic macrophage, or whether TNF-α may be produced by other cell types in CAC, including T cells and epithelial cells (99). Additionally, a very interesting study demonstrated that TNF-α expression is elevated in CAC carcinogenesis and genetic inactivation of the type 1 TNF receptor (TNFR1) or TNF signaling inhibition with a soluble decoy receptor reduced CAC promotion (100). Moreover, the dependence of TNF-α to carcinogenesis in a distinct model of CAC than AOM + DSS, as T-bet deficiency was observed in dendritic cells, reinforces its importance in CAC tumorigenesis (101). Thus, the same prototypical cytokines, TNF-α and IL-6, which are increased in obesity-associated inflammation, have been found to be crucial in promoting colitis induced cancer.
Obesity-associated inflammation is clearly not restricted to adipocytes but disseminated in all metabolic tissues (51,52,102-104). Furthermore, it was recently observed that non-metabolic glandular organs, including colon, also present signs of low-grade inflammation in obesity (105-109). Importantly, TNF-α overexpression was consistently elevated in colons of genetically- or diet-induced obesity rodents (106-109). Congruent with an increased inflammatory response IL-6 and other cytokines are also upregulated in the colons of obese animals (110,111) suggesting that the obese colonic tissue recapitulates the inflammatory timbre constantly observed in metabolic tissues of obese individuals. Accordingly, obese Zucker rats treated with azoxymethane (AOM) manifested higher incidence of tubular adenomas and TNF-α than their lean matched controls (112). Recently, it was observed that leptin deficient and high fat diet fed mice exposed to a combination of AOM + DSS developed higher colonic inflammation than their lean counterparts and increased colonic adenoma numbers in a TNF-α dependent manner (109). Importantly, treatment with infliximab, a monoclonal antibody that neutralizes TNF-α, inhibited the activation of colonic JNK and IKK resulting in the decreased quantity of colonic adenoma and the growth of colon cancer xenografts (109). Interestingly, enhanced production of IL-6 and TNF-α was also observed in a hepatocarcinoma (HCC) mouse model (113). In these animals HFD induced increased expression of TNF-α and ablation of TNFR1 significantly reduced obesity-enhanced HCC development (113). Altogether, these studies suggest that the inflammatory milieu instigated by obesity may be a general mechanism that links obesity to gastrointestinal cancers.
Activation of IKK/NF-κB pathway is consistently associated with both colitis- and obesity-associated carcinogenesis (99,109,113,114). Interestingly, the outcome of TNF mediated NF-κB activation, considering target gene expression, may alternate, depending on the tissue or cell type stimulated. In this context, NF-κB exerts not only intrinsic effects within pre-malignant epithelial cells, but also modulates actions of infiltrating lymphocytes and macrophages (115,116). In normal physiology, NF-κB response is self-limited by the induction of negative feedback loops (117,118). However in chronic inflammation induced by obesity, continuous cytokine release by immune cells of the stromal vascular fraction results in sustained IKK activation, which deregulates NF-κB activity (109).
The pro-oncogenic effects of NF-κB involve other intracellular mechanisms, besides continuous activation of IKK. Transcription factors, including STAT3, may play a role in NF-κB dependent tumorigenesis (7). In tumors, accumulation of the prototypical NF-κB complex (p50/RelA) in the cellular nucleus is regulated through acetylation by p300 (119,120). It is relevant that STAT3 though p300 mediates RelA acetylation to promote and sustain NF-κB activity (121). Importantly, cytokines and growth factors encoded by NF-κB target genes, especially IL-6, are critical STAT3 activators (122-124). Interestingly, other inflammatory cytokines, such as IL-17, promotes STAT3 activation through NF-κB mediated IL-6 expression (125,126). Congruent with this data, expression of several inflammatory mediators, such as IL-6, COX2, IL-17 and IL-23, is also dependent of STAT3 as a RelA co-transcriptional factor (127-130).
Investigations on the influence of IL-6 in CAC showed that knockout mice for this cytokine developed less and smaller colonic adenomas than controls in a CAC model (123). Moreover, pharmacological inhibition of the common signaling receptor gp130 by a soluble gp130-Fc fusion protein also resulted in decreased tumor number and size in animals exposed to a CAC model (131). In consonance, genetic activation of gp130 in enterocytes of mice in a CAC model promoted increased tumor number and growth (132) whereas STAT3 deletion in intestinal epithelial cells markedly decreased the incidence and volume of AOM + DSS induced tumors (123). IL-6 is mostly produced by myeloid cells, primarily by lamina propria macrophages and dendritic cells during tumor initiation and by T cells during tumor progression, in CAC models (123,131,133). This is probably a consequence of the high inflammatory activity of CAC tumors and the continuous injury and death of enterocytes during tumor development (123). In other words, epithelial cells and cancer cells, as well as tumor-associated fibroblasts can also produce IL-6 and may contribute to the total amount of this cytokine, particularly in sporadic colorectal and obesity-associated colorectal cancers.
Taken together, these data provide strike evidence for the involvement of TNF-α by promoting continuous stimulation of IKK/NF-κB pathway in the pathogenesis of obesity-associated colon cancers. Furthermore, interactions between IL-6, STAT3 and NF-κB may have a role in this phenomenon.
TNF-α influence on obesity-associated colon carcinogenesis phases
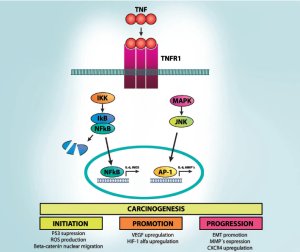
Carcinogenesis can be didactically divided into three mechanistic phases: initiation (which involves stable genomic alterations), promotion (which involves the proliferation of genetically altered cells) and progression (which involves an increase in tumor size, its spreading and acquisition of additional genetic changes) (134). Notably, TNF-α may influence all those stages of tumor development (Figure 2).
Initiation
More than six decades ago, Peyton Rous defined initiation phase as a “subthreshold neoplastic state”, in which “latent tumor cells” wait for the promotion stimuli to proliferate (134-136). Since the majority of cancers need at least 4-5 mutations to acquire a neoplastic phenotype (26,137) the initiation phase in current words corresponds to the early mutations observed in premalignant cells. TNF-α modulates the initiation phase by at least three mechanisms. First, TNF-α released by inflammatory cells in the tumor microenvironment may induce reactive oxygen and nitrogen species (RNOS) in adjacent epithelial cells, inducing DNA damage and genomic instability (138,139). Second, colorectal tumors may be initiated by increased activity of Wnt/β-catenin signaling in colon progenitor cells (140-142). Importantly, TNF-α through activation of NF-κB or repression of GSK3β promotes Wnt/β-catenin signaling in gastrointestinal mucosa (143,144). Finally, NF-κB regulates several tumor suppressor pathways; specifically it inhibits p53 activity through competition for the p300 and CBP co-activator proteins (145,146).
In spite of the effects of TNF-α in a number of important molecules involved in tumoral initiation, experimental evidence from obese Zucker rats and high fatty diet fed mice demonstrate that treatment with AOM does not changed the total number of aberrant crypt foci (147,148). Furthermore, recent data showed that obese individuals have an increased risk to develop β-catenin negative colon cancer, but not β-catenin positive (149). Overall, these findings are consistent with minor effects of obesity low-grade inflammation on the colonic tumor initiation.
Promotion
Initiation is an irreversible process, whereas promotion may be modulated by the stimuli intensity and even reversible if the stimuli are removed (134-136). The promotion phase is characterized by increased cell proliferation and reduced cell death. It may be an early or late event in tumor development, as late proliferation of dormant malignant lesions may also occur (150). Evidence for TNF-α-mediated colonic adenoma promotion in obesity came from observing elevated numbers and larger tumors size in obese animals compared to their lean controls, which was associated to IKK overexpression in these tumors (109). Accordingly, neutralization of TNF-α reverted the growth rate of colon cancer xenograft implanted in high fat diet fed animals to lean settings (109). Furthermore, obese animals switched from a HFD to regular chow after carcinogen exposure developed more tumors than lean controls, but similar number of aberrant crypt foci, the colonic pre-neoplastic lesion (148).
During tumor promotion, it is necessary to increase tumoral blood supply, mainly by angiogenesis triggered by tumor hypoxia (151). Interestingly, activation of NF-κB, STAT3 and AP-1 in tumoral microenvironment cells, such as tumor-associated macrophages (TAMs) and fibroblasts directly regulate important pro-angiogenic genes, including IL-8, CXCL1, CXCL8, VEGF, and hypoxia inducible factor 1 alpha (HIF1α) (152-154). Inactivation of NF-κB or STAT3, neutralization of CCL2 or CXCL12, or TAM depletion leads to ineffective angiogenesis and reduced tumor growth. Interestingly, the visceral adipose tissue of patients with colon cancer presents concomitantly increases in TNF-α and the pro-angiogenic factors, such as HIF1α and VEGF (155). Altogether, these data indicate that obesity-associated inflammation strongly affects colon cancer promotion phase.
Progression
Metastatic disease is the most critical feature of cancer in a clinical setting as it is responsible for over 90% of disease mortality (156). The process of invasion and metastasis can be schematically divided into four major steps. First, epithelial-mesenchimal transition (EMT) is required for acquisition of a fibroblastoid phenotype by an epithelial malignant cell, resulting in increased motility and capacity to invade basal membranes and reach blood vessels or lymphatics (157). Second, cancer cells intravasate into blood vessels and lymphatics, with possible involvement of cytokines and inflammatory effectors by promoting increased vascular permeability (158,159). Third, metastatic cells should survive and travel in circulation (158,159). Fourth, circulating cancer cells should adhere and extravasate in a distant site, in which they need to interact with immune, inflammatory, and stromal cells to proliferate (158,159). Some of these cells may already be targeted to a pre-metastatic niche, in which soluble growth factors secreted by the primary tumor prime certain tissues for tumor cell engraftment, known as ‘metastatic niche’ theory (160-162). Obesity is associated not only with an increased incidence of colon cancer, but also with a more aggressive natural history; the patients are younger, present more metastasis to lymph nodes and the disease free and overall survival are reduced (163). In spite of the lack of direct evidence that obesity-associated inflammation interferes in these endpoints, TNF-α may exert effects in all metastatic phases.
TNF-α may contribute to cell migration-promoting EMT through stabilization of Snail, an inhibitor of E-cadherin expression, a key event in EMT (164-166). Interestingly, TNF-α, through NF-κB signaling, can also induce overexpression of other important regulators of EMT such as Twist, ZEB1 and SLUG, contributing to its induction (165,167-169). Another mechanism by which TNF-α can induce EMT is through synergistic action with transforming growth factor β1 (TGFβ) (170,171). Importantly, in a model of colon cancer, cancer cell invasiveness was associated to extracellular matrix proteolysis, a process that is dependent of matrix metalloproteinases (MMP) release, which may also be regulated by TNF-α induced activation of NF-κB (172,173).
After intravasation in circulation, metastatic cells need to survive in suspension and resist detachment-induced death, named anoikis (174). Notably, TNF-α, and other cytokines can promote survival of circulating metastatic cells, through activation of NF-κB in either inflammatory and cancer cells or by promoting a physical link between cancer cells and TAMs, allowing them to travel together throughout the circulation and evading immunological attacks (175,176). Furthermore, migration of metastatic cells is directed by chemokine gradients that are sensed by many receptors, including CXCR4, which expression is upregulated by TNF-α (177).
In a distant site, circulating metastatic cells are arrested on the endothelium in an integrin-dependent process. Therefore, adhesion between malignant and endothelial cells are important mediators of this process (175). Importantly, bone marrow-derived haematopoietic cells that express vascular endothelial growth factor (VEGF) receptor 1 (VEGFR) migrate and determine the metastatic sites before the arrival of neoplastic cells (160). Interestingly, the pre-metastatic niche is also defined by the tumor-secreted matrix protein versican, which activates TLR2 on host macrophages and promotes release of TNF-α (178). Accordingly, metastasis formation was dramatically reduced, by TLR2 or TNF-α suppression (178). Furthermore, VEGFA, TGFβ, and TNF-α secreted by the primary tumor promoted the expression of inflammatory proteins S100A8 and S100A9, leading to infiltration of lungs, the target site of metastasis, by myeloid cells expressing the cell surface antigens integrin αM (also known as MAC1) or CD11b (161). As a result, treatment with S100A8 and S100A9 antibodies diminished infiltration of MAC1 myeloid cells, resulting in a remarkable reduction in metastasis incidence (161). Specifically in regard to colon cancer, it was observed that targeting VEGF2 and other cytokines involved in the pre-metastatic niche formation reduced liver metastasis formation (179).
Conclusions
Recent clinical and experimental data provide support for the involvement of TNF-α in the pathogenesis of obesity-associated colon cancer. TNF-α promotes colon cancer in obese states through direct effects on premalignant cells and by orchestrating a tumor-promoting microenvironment through actions on several distinct cell types. However, how the cellular component of obese adipose tissue microenvironment promotes a “fertile soil” to carcinogenesis and whether interactions between inflammatory cells and adipocytes contribute to promotion and progression of cancer is still largely unknown. Since these studies may contribute to a better understanding of carcinogenesis in general and give clues to cancer treatment, it will be critical in the future to systematically evaluate how an obesity-associated inflammatory microenvironment contributes to colon carcinogenesis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Majno G, Joris I. Cells, tissues, and disease: principles of general pathology. New York: Oxford University Press, 2004.
- Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454:428-35. [PubMed]
- Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 2013;339:172-7. [PubMed]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860-7. [PubMed]
- Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012;32:2045-51. [PubMed]
- Glass CK, Saijo K, Winner B, et al. Mechanisms underlying inflammation in neurodegeneration. Cell 2010;140:918-34. [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [PubMed]
- Thomas L. The lives of a cell; notes of a biology watcher. New York: Viking Press, 1974.
- Scrivo R, Vasile M, Bartosiewicz I, et al. Inflammation as “common soil” of the multifactorial diseases. Autoimmun Rev 2011;10:369-74. [PubMed]
- Nathan C, Ding A. Nonresolving inflammation. Cell 2010;140:871-82. [PubMed]
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell 2010;140:771-6. [PubMed]
- Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science 2013;339:166-72. [PubMed]
- Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol 2008;8:279-89. [PubMed]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol 2008;8:349-61. [PubMed]
- Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol 2010;10:427-39. [PubMed]
- Levy BD, Clish CB, Schmidt B, et al. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol 2001;2:612-9. [PubMed]
- Maddox JF, Hachicha M, Takano T, et al. Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem 1997;272:6972-8. [PubMed]
- József L, Zouki C, Petasis NA, et al. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-kappa B and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc Natl Acad Sci U S A 2002;99:13266-71. [PubMed]
- Arita M, Ohira T, Sun YP, et al. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol 2007;178:3912-7. [PubMed]
- Bellingan GJ, Caldwell H, Howie SE, et al. In vivo fate of the inflammatory macrophage during the resolution of inflammation: inflammatory macrophages do not die locally, but emigrate to the draining lymph nodes. J Immunol 1996;157:2577-85. [PubMed]
- Fadok VA, Bratton DL, Konowal A, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 1998;101:890-8. [PubMed]
- Odegaard JI, Chawla A. The immune system as a sensor of the metabolic state. Immunity 2013;38:644-54. [PubMed]
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 2013;339:286-91. [PubMed]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986;315:1650-9. [PubMed]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39-51. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 1993;259:87-91. [PubMed]
- Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796-808. [PubMed]
- Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821-30. [PubMed]
- Johnson AM, Olefsky JM. The origins and drivers of insulin resistance. Cell 2013;152:673-84. [PubMed]
- Bergeron V, Vienne A, Sokol H, et al. Risk factors for neoplasia in inflammatory bowel disease patients with pancolitis. Am J Gastroenterol 2010;105:2405-11. [PubMed]
- von Roon AC, Reese G, Teare J, et al. The risk of cancer in patients with Crohn’s disease. Dis Colon Rectum 2007;50:839-55. [PubMed]
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427-34. [PubMed]
- Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011;365:1713-25. [PubMed]
- Cario E, Rosenberg IM, Brandwein SL, et al. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol 2000;164:966-72. [PubMed]
- Hisamatsu T, Suzuki M, Reinecker HC, et al. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 2003;124:993-1000. [PubMed]
- Yoshida M, Kobayashi K, Kuo TT, et al. Neonatal Fc receptor for IgG regulates mucosal immune responses to luminal bacteria. J Clin Invest 2006;116:2142-2151. [PubMed]
- Neish AS, Gewirtz AT, Zeng H, et al. Prokaryotic regulation of epithelial responses by inhibition of IkappaB-alpha ubiquitination. Science 2000;289:1560-3. [PubMed]
- Kelly D, Campbell JI, King TP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol 2004;5:104-12. [PubMed]
- Diehl GE, Longman RS, Zhang JX, et al. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 2013;494:116-20. [PubMed]
- Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin 2010;60:207-21. [PubMed]
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. [PubMed]
- Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med 2007;356:213-5. [PubMed]
- Flegal KM, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491-7. [PubMed]
- Filozof C, Gonzalez C, Sereday M, et al. Obesity prevalence and trends in Latin-American countries. Obes Rev 2001;2:99-106. [PubMed]
- Abegunde DO, Mathers CD, Adam T, et al. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet 2007;370:1929-38. [PubMed]
- Yoon KH, Lee JH, Kim JW, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006;368:1681-8. [PubMed]
- Rask-Madsen C, Kahn CR. Tissue-specific insulin signaling, metabolic syndrome, and cardiovascular disease. Arterioscler Thromb Vasc Biol 2012;32:2052-9. [PubMed]
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell 2012;148:852-71. [PubMed]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006;116:1793-801. [PubMed]
- Cai D, Yuan M, Frantz DF, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med 2005;11:183-90. [PubMed]
- Itani SI, Ruderman NB, Schmieder F, et al. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002;51:2005-11. [PubMed]
- Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature 2002;420:333-6. [PubMed]
- Arkan MC, Hevener AL, Greten FR, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 2005;11:191-8. [PubMed]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175-84. [PubMed]
- Aguirre V, Werner ED, Giraud J, et al. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 2002;277:1531-7. [PubMed]
- Gao Z, Hwang D, Bataille F, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem 2002;277:48115-21. [PubMed]
- Carvalho-Filho MA, Ueno M, Hirabara SM, et al. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes 2005;54:959-67. [PubMed]
- Ropelle ER, Pauli JR, Cintra DE, et al. Targeted disruption of inducible nitric oxide synthase protects against aging, S-nitrosation, and insulin resistance in muscle of male mice. Diabetes 2013;62:466-70. [PubMed]
- Sugita H, Fujimoto M, Yasukawa T, et al. Inducible nitric-oxide synthase and NO donor induce insulin receptor substrate-1 degradation in skeletal muscle cells. J Biol Chem 2005;280:14203-11. [PubMed]
- Yasukawa T, Tokunaga E, Ota H, et al. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem 2005;280:7511-8. [PubMed]
- Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013;496:445-55. [PubMed]
- Kamei N, Tobe K, Suzuki R, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem 2006;281:26602-14. [PubMed]
- Kanda H, Tateya S, Tamori Y, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest 2006;116:1494-505. [PubMed]
- Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006;116:115-24. [PubMed]
- Gerhardt CC, Romero IA, Cancello R, et al. Chemokines control fat accumulation and leptin secretion by cultured human adipocytes. Mol Cell Endocrinol 2001;175:81-92. [PubMed]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003;3:23-35. [PubMed]
- Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011;332:243-7. [PubMed]
- Han MS, Jung DY, Morel C, et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 2013;339:218-22. [PubMed]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007;447:1116-20. [PubMed]
- Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab 2008;7:496-507. [PubMed]
- Bouhlel MA, Derudas B, Rigamonti E, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 2007;6:137-43. [PubMed]
- Oh DY, Talukdar S, Bae EJ, et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010;142:687-98. [PubMed]
- Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med 2012;18:363-74. [PubMed]
- Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009;15:921-9. [PubMed]
- Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009;15:914-20. [PubMed]
- Winer DA, Winer S, Shen L, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med 2011;17:610-7. [PubMed]
- Molofsky AB, Nussbaum JC, Liang HE, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 2013;210:535-49. [PubMed]
- Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 2009;15:930-9. [PubMed]
- Lynch L, Nowak M, Varghese B, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity 2012;37:574-87. [PubMed]
- Schipper HS, Rakhshandehroo M, van de Graaf SF, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest 2012;122:3343-54. [PubMed]
- Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012;489:242-9. [PubMed]
- Holmes E, Li JV, Marchesi JR, et al. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab 2012;16:559-64. [PubMed]
- Caesar R, Reigstad CS, Backhed HK, et al. Gut-derived lipopolysaccharide augments adipose macrophage accumulation but is not essential for impaired glucose or insulin tolerance in mice. Gut 2012;61:1701-7. [PubMed]
- Carvalho BM, Guadagnini D, Tsukumo DM, et al. Modulation of gut microbiota by antibiotics improves insulin signalling in high-fat fed mice. Diabetologia 2012;55:2823-34. [PubMed]
- Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761-72. [PubMed]
- Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008;57:1470-81. [PubMed]
- Oliveira AG, Carvalho BM, Tobar N, et al. Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. Diabetes 2011;60:784-96. [PubMed]
- Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007;56:1986-98. [PubMed]
- Shi H, Kokoeva MV, Inouye K, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116:3015-25. [PubMed]
- Caricilli AM, Picardi PK, de Abreu LL, et al. Gut microbiota is a key modulator of insulin resistance in TLR 2 knockout mice. PLoS Biol 2011;9:e1001212. [PubMed]
- Okin D, Medzhitov R. Evolution of inflammatory diseases. Curr Biol 2012;22:R733-40. [PubMed]
- Calle EE, Thun MJ, Petrelli JM, et al. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 1999;341:1097-105. [PubMed]
- Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev 2010;11:19-30. [PubMed]
- Braegger CP, Nicholls S, Murch SH, et al. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet 1992;339:89-91. [PubMed]
- Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066-78. [PubMed]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009;9:361-71. [PubMed]
- Terzić J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology 2010;138:2101-2114.e5.
- Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004;118:285-96. [PubMed]
- Popivanova BK, Kitamura K, Wu Y, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 2008;118:560-70. [PubMed]
- Garrett WS, Punit S, Gallini CA, et al. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell 2009;16:208-19. [PubMed]
- De Souza CT, Araujo EP, Bordin S, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 2005;146:4192-9. [PubMed]
- Ropelle ER, Flores MB, Cintra DE, et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKbeta and ER stress inhibition. PLoS Biol 2010;8:e1000465. [PubMed]
- Zhang X, Zhang G, Zhang H, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008;135:61-73. [PubMed]
- Subbaramaiah K, Howe LR, Bhardwaj P, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329-46. [PubMed]
- Liu Z, Brooks RS, Ciappio ED, et al. Diet-induced obesity elevates colonic TNF-alpha in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J Nutr Biochem 2012;23:1207-13. [PubMed]
- Kubota M, Shimizu M, Sakai H, et al. Renin-angiotensin system inhibitors suppress azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Biochem Biophys Res Commun 2011;410:108-13. [PubMed]
- Yasuda Y, Shimizu M, Shirakami Y, et al. Pitavastatin inhibits azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Cancer Sci 2010;101:1701-7. [PubMed]
- Flores MB, Rocha GZ, Damas-Souza DM, et al. Obesity-induced increase in tumor necrosis factor-α leads to development of colon cancer in mice. Gastroenterology 2012;143:741-53.e1-4.
- Padidar S, Farquharson AJ, Williams LM, et al. High-fat diet alters gene expression in the liver and colon: links to increased development of aberrant crypt foci. Dig Dis Sci 2012;57:1866-74. [PubMed]
- Mentor-Marcel RA, Bobe G, Barrett KG, et al. Inflammation-associated serum and colon markers as indicators of dietary attenuation of colon carcinogenesis in ob/ob mice. Cancer Prev Res (Phila) 2009;2:60-9. [PubMed]
- Jain SS, Bird RP. Elevated expression of tumor necrosis factor-alpha signaling molecules in colonic tumors of Zucker obese (fa/fa) rats. Int J Cancer 2010;127:2042-50. [PubMed]
- Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 2010;140:197-208. [PubMed]
- Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461-6. [PubMed]
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005;7:211-7. [PubMed]
- Ammirante M, Luo JL, Grivennikov S, et al. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 2010;464:302-5. [PubMed]
- Paszek P, Ryan S, Ashall L, et al. Population robustness arising from cellular heterogeneity. Proc Natl Acad Sci U S A 2010;107:11644-9. [PubMed]
- Ashall L, Horton CA, Nelson DE, et al. Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science 2009;324:242-6. [PubMed]
- Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J 2002;21:6539-48. [PubMed]
- Lf Chen. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 2001;293:1653-7. [PubMed]
- Lee H, Herrmann A, Deng JH, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell 2009;15:283-93. [PubMed]
- Bollrath J, Greten FR. IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep 2009;10:1314-9. [PubMed]
- Grivennikov S, Karin E, Terzic J, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009;15:103-13. [PubMed]
- Darnell JE Jr. STATs and gene regulation. Science 1997;277:1630-5. [PubMed]
- Wang L, Yi T, Kortylewski M, et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med 2009;206:1457-64. [PubMed]
- Ogura H, Murakami M, Okuyama Y, et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 2008;29:628-36. [PubMed]
- Kortylewski M, Xin H, Kujawski M, et al. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell 2009;15:114-23. [PubMed]
- Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol 2004;5:392-401. [PubMed]
- Wang J, Wang X, Hussain S, et al. Distinct roles of different NF-kappa B subunits in regulating inflammatory and T cell stimulatory gene expression in dendritic cells. J Immunol 2007;178:6777-88. [PubMed]
- Dalwadi H, Krysan K, Heuze-Vourc’h N, et al. Cyclooxygenase-2-dependent activation of signal transducer and activator of transcription 3 by interleukin-6 in non-small cell lung cancer. Clin Cancer Res 2005;11:7674-82. [PubMed]
- Matsumoto S, Hara T, Mitsuyama K, et al. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J Immunol 2010;184:1543-51. [PubMed]
- Bollrath J, Phesse TJ, von Burstin VA, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 2009;15:91-102. [PubMed]
- Becker C, Fantini MC, Schramm C, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 2004;21:491-501. [PubMed]
- Foulds L. The natural history of cancer. J Chronic Dis 1958;8:2-37. [PubMed]
- Friedewald WF, Rous P. The Initiating and Promoting Elements in Tumor Production: An Analysis of the Effects of Tar, Benzpyrene, and Methylcholanthrene on Rabbit Skin. J Exp Med 1944;80:101-26. [PubMed]
- Friedewald WF, Rous P. The pathogenesis of deferred cancer; a study of the after-effects of methylcholanthrene upon rabbit skin. J Exp Med 1950;91:459-84. [PubMed]
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759-67. [PubMed]
- Meira LB, Bugni JM, Green SL, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest 2008;118:2516-25. [PubMed]
- Shaked H, Hofseth LJ, Chumanevich A, et al. Chronic epithelial NF-kappaB activation accelerates APC loss and intestinal tumor initiation through iNOS up-regulation. Proc Natl Acad Sci U S A 2012;109:14007-12. [PubMed]
- Korinek V, Barker N, Morin PJ, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 1997;275:1784-7. [PubMed]
- Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997;275:1787-90. [PubMed]
- Barker N, Ridgway RA, van Es JH, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009;457:608-11. [PubMed]
- Oguma K, Oshima H, Aoki M, et al. Activated macrophages promote Wnt signalling through tumour necrosis factor-alpha in gastric tumour cells. EMBO J 2008;27:1671-81. [PubMed]
- Umar S, Sarkar S, Wang Y, et al. Functional cross-talk between beta-catenin and NFkappaB signaling pathways in colonic crypts of mice in response to progastrin. J Biol Chem 2009;284:22274-84. [PubMed]
- Wadgaonkar R, Phelps KM, Haque Z, et al. CREB-binding protein is a nuclear integrator of nuclear factor-kappaB and p53 signaling. J Biol Chem 1999;274:1879-82. [PubMed]
- Ravi R, Mookerjee B, van Hensbergen Y, et al. p53-mediated repression of nuclear factor-kappaB RelA via the transcriptional integrator p300. Cancer Res 1998;58:4531-6. [PubMed]
- Raju J, Bird RP. Energy restriction reduces the number of advanced aberrant crypt foci and attenuates the expression of colonic transforming growth factor beta and cyclooxygenase isoforms in Zucker obese (fa/fa) rats. Cancer Res 2003;63:6595-601. [PubMed]
- Tuominen I, Al-Rabadi L, Stavrakis D, et al. Diet-induced obesity promotes colon tumor development in azoxymethane-treated mice. PLoS One 2013;8:e60939. [PubMed]
- Morikawa T, Kuchiba A, Lochhead P, et al. Prospective analysis of body mass index, physical activity, and colorectal cancer risk associated with beta-catenin (CTNNB1) status. Cancer Res 2013;73:1600-10. [PubMed]
- Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 2007;7:834-46. [PubMed]
- Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer 2008;8:967-75. [PubMed]
- Murdoch C, Muthana M, Coffelt SB, et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008;8:618-31. [PubMed]
- Kujawski M, Kortylewski M, Lee H, et al. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest 2008;118:3367-77. [PubMed]
- Rius J, Guma M, Schachtrup C, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 2008;453:807-11. [PubMed]
- Catalán V, Gómez-Ambrosi J, Rodríguez A, et al. Up-regulation of the novel proinflammatory adipokines lipocalin-2, chitinase-3 like-1 and osteopontin as well as angiogenic-related factors in visceral adipose tissue of patients with colon cancer. J Nutr Biochem 2011;22:634-41. [PubMed]
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell 2006;127:679-95. [PubMed]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420-8. [PubMed]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002;2:563-72. [PubMed]
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer 2006;6:449-58. [PubMed]
- Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005;438:820-7. [PubMed]
- Hiratsuka S, Watanabe A, Aburatani H, et al. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 2006;8:1369-75. [PubMed]
- Hiratsuka S, Watanabe A, Sakurai Y, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol 2008;10:1349-55. [PubMed]
- Sinicrope FA, Foster NR, Sargent DJ, et al. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res 2010;16:1884-93. [PubMed]
- Wu K, Bonavida B. The activated NF-kappaB-Snail-RKIP circuitry in cancer regulates both the metastatic cascade and resistance to apoptosis by cytotoxic drugs. Crit Rev Immunol 2009;29:241-54. [PubMed]
- Min C, Eddy SF, Sherr DH, et al. NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem 2008;104:733-44. [PubMed]
- Wang H, Wang HS, Zhou BH, et al. Epithelial-mesenchymal transition (EMT) induced by TNF-alpha requires AKT/GSK-3beta-mediated stabilization of snail in colorectal cancer. PLoS One 2013;8:e56664. [PubMed]
- Storci G, Sansone P, Mari S, et al. TNFalpha up-regulates SLUG via the NF-kappaB/HIF1alpha axis, which imparts breast cancer cells with a stem cell-like phenotype. J Cell Physiol 2010;225:682-91. [PubMed]
- Chua HL, Bhat-Nakshatri P, Clare SE, et al. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene 2007;26:711-24. [PubMed]
- Maier HJ, Schmidt-Strassburger U, Huber MA, et al. NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer Lett 2010;295:214-28. [PubMed]
- Borthwick LA, Gardner A, De Soyza A, et al. Transforming Growth Factor-β1 (TGF-β1) Driven Epithelial to Mesenchymal Transition (EMT) is Accentuated by Tumour Necrosis Factor α (TNFα) via Crosstalk Between the SMAD and NF-κB Pathways. Cancer Microenviron 2012;5:45-57. [PubMed]
- Takahashi E, Nagano O, Ishimoto T, et al. Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J Biol Chem 2010;285:4060-73. [PubMed]
- Kitamura T, Taketo MM. Keeping out the bad guys: gateway to cellular target therapy. Cancer Res 2007;67:10099-102. [PubMed]
- Kitamura T, Kometani K, Hashida H, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet 2007;39:467-75. [PubMed]
- Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol 2001;13:555-62. [PubMed]
- Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 2009;9:274-84. [PubMed]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006;124:263-6. [PubMed]
- Zhao C, Lu X, Bu X, et al. Involvement of tumor necrosis factor-alpha in the upregulation of CXCR4 expression in gastric cancer induced by Helicobacter pylori. BMC Cancer 2010;10:419. [PubMed]
- Kim S, Takahashi H, Lin WW, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009;457:102-6. [PubMed]
- Yamamoto M, Kikuchi H, Ohta M, et al. TSU68 prevents liver metastasis of colon cancer xenografts by modulating the premetastatic niche. Cancer Res 2008;68:9754-62. [PubMed]


